185
advertisement

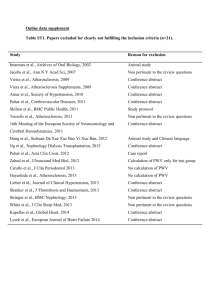
The correlation between vascular diseases and periodontal diseases – a literature review Agnieszka Kręgielczak, Marzena Wyganowska-Świątkowska, Janina Stopa Department of Conservative Dentistry and Periodontology, Poznań University of Medical Sciences, Poland ul. Bukowska 70, Poznań, Poland Head of the Clinic: Prof. Dr Hab. Janina Stopa, MD, PhD Corresponding author: Dr n. med. Agnieszka Kręgielczak, MD, PhD ul. Szczawiowa 9, 61-680 Poznań Phone: 501 587 670 Fax: 61 853 70 27 akregiel@ump.edu.pl Abstract Introduction The issues concerning the correlation between periodontal diseases and vascular diseases are the subject of continuous discussion in literature. Chronic periodontitis has been recognised as one of the risk factors of cardiovascular diseases such as: atherosclerosis, endocarditis, myocardial infarction or even aortic aneurysms. A particular role is ascribed to the inflammatory factors participating in the aetiopathogenesis of both vascular and periodontal diseases. Aim of study An evaluation of periodontal diseases as a risk factor of vascular diseases with special attention given to the role of periopathogens in the development of atherosclerosis and aneurysms. Summary The mechanisms of correlation between the occurrence of periodontal diseases and atherosclerosis and aortic aneurysms were described on the basis of available literature. The analysis of clinical, epidemiological and experimental studies confirmed the correlation between the diseases in question. Key words: periodontitis, atherosclerosis, aneurysms, periopathogens Introduction Diseases of the cardiovascular system are the most frequent cause of deaths in countries with well-developed health care. The diseases mainly result from the atherosclerotic reconstruction of the arterial system. There is a continuous search for other risk factors responsible for the occurrence of vascular diseases than those that have been well investigated so far. On the basis of the published results of clinical and epidemiological examinations it has been observed that chronic inflammatory processes occurring in the oral cavity may be a risk factor responsible for the occurrence of vascular diseases.1,2 Statistically significant correlations between the poor state of health of the oral cavity and the occurrence of certain systemic diseases have been proved, especially endocarditis, coronary artery atherosclerosis, carotid artery atherosclerosis, abdominal aortic atherosclerosis, myocardial infarction and aortic aneurysms.1,2,3 Along with caries, chronic periodontitis is considered to be the most frequent pathological process within the oral cavity. Periodontal pockets, which develop in the course of those diseases, are a perfect niche for microorganisms, especially for anaerobic bacilli, which are strongly pathogenic. It has been proved that bacteria, bacterial products and inflammatory and immunological mediators which are present in the pathologically changed periodontis may act outside the oral cavity and the pathogens from the periodontis may be an inflammatory factor participating in the aetiopathogenesis of vascular diseases related with atherosclerotic processes.4 The problem has been investigated by many authors who published both numerous review papers and research projects. Aim The aim of this study is an evaluation of the correlation between periodontal diseases and vascular diseases with special attention given to the role of periopathogens in the aetiopathogenesis of arterial atherosclerosis and aneurysms. On the basis of numerous studies subgingival plaque bacteria in patients with chronic periodontitis were found to be the possible cause of bacteraemia. The bacteria may enter the bloodstream through the epithelium of gingival pockets, whose area is equal to the area of the human palm.5 Scannapienco6 thinks that bacteraemia in the organism may be caused not only by chronic periodontitis but also by all types of dental treatment or even by everyday hygienic procedures. The report by the American Academy of Periodontology of 1999, published in Journal of Periodontology in 2000, states that periodontal diseases may be related with diabetes, premature births, cardiovascular diseases, joint diseases and pulmonary diseases.1, 3, 7 It was also found that in comparison with healthy people patients with certain systemic diseases are more susceptible to the occurrence of periodontal diseases. Those diseases include: diabetes, AIDS, osteoporosis, Crohn's disease, Papillon-Lefevre syndrome, Down syndrome or even peptic ulcer disease. There are several mechanisms accounting for the possible influence of periodontitis on the general state of health. According to Scannapienco6 they are: (1) spreading of the infection from the periodontis into deeper located adjacent tissues; (2) the passage of inflammatory mediators into the bloodstream and their influence on the functions of other organs; (3) the penetration of bacteria from the oral cavity into the bloodstream and causing inflammations in distant locations; (4) spreading of bacteria and their products through the mucosa into distant organs. The first scientific reports discussing the issue of correlation between periodontitis and cardiovascular diseases appeared in the 1980s and were based on numerous epidemiological studies.8, 9, 10 Syrjanen et al11 in their publication of 1989 proved the dependence between the intensification of the periodontal disease and the frequency of incidence of stroke. The studies by Matilla et al10 of 1989 and 1999 confirmed the concomitance of periodontal diseases and infarctions in men. De Stefano7 in an analysis of 9760 people aged 25-74 years proved an increased risk of occurrence of coronary disease by 25% in people with diagnosed periodontal disease in comparison with the group with healthy periodontium. He also proved that the risk of occurrence of coronary disease in men aged over 50 years with diagnosed periodontal disease increases by 70% in comparison with healthy men.7 Elter9 on the basis of a research on 6436 people found more frequent occurrences of infarcts and coronary disease in patients with chronic periodontitis in comparison with a group of people with healthy periodontium. He observed particularly frequent occurrences of vascular diseases in toothless people, proving the correlations between the number of lost teeth and the occurrence of vascular diseases.9 The correlation between the occurrence of advanced periodontal diseases and carotid artery atherosclerosis was also confirmed by Beck et al.1, 2 On the other hand, the Finnish scientists noticed that the number of lost teeth is a statistically significant risk factor for the occurrence of coronary artery disease in smokers. 12 Besides, the authors proved the correlation between the occurrence of moderate and severe periodontitis and the thickening of the middle layer of the carotid artery wall. As results from the presented overview of the literature, most authors investigating the problem confirm the correlation between the occurrence of vascular diseases and periodontal diseases. However, some authors have the opposite point of view and find no correlation between the number of teeth in the oral cavity, the state of the periodontis and coronary artery disease.13 The WHO reports of 1994 and 1999 mention a rather coincidental relation between periodontitis and atherosclerosis. However, it is necessary to remember that the studies which made the material included in the reports were made in Scandinavia, where most patients’ periodontis is in good state.13 Atherosclerosis is a process which consists in local widening of an arterial vessel wall by thickening of the internal lining layer and the middle layer, consisting of smooth muscles, collagen and elastic fibres.14 According to Ross14 the first stage of formation of atherosclerotic plaque is adhesion of monocytes to the arterial vessel endothelium. This process is regulated by adhesins located on the surface of endothelial cells of vessels (ICAM-1, ELAM-1, VCAM1). The function of adhesins is influenced by numerous factors, including: lipopolysaccharides, prostaglandins and inflammatory cytokines. Lipopolysaccharides are endotoxins which may come from bacterial cells in the periodontitis, whereas prostaglandins and inflammatory cytokines develop as a result of the immune response which may also be initiated by periopathogens. In response to bacterial infection monocytes become activated, penetrate into the middle layer of the vessel and absorb low density lipoproteins (LDL) and thus transform them into foam cells, of which atherosclerotic plaque is composed. In the middle layer of the vessel monocytes may also transform into macrophages and produce inflammatory cytokines: interleukin IL-1β, TNF-α, prostaglandin 2. This will initiate the development of damage to the vessel wall. Additionally, the fibroblast growth factor and plaque growth factor stimulate the proliferation of smooth muscles and collagen within the middle layer of the vessel causing it to thicken. Thus, the vessel lumen is narrowed and the arterial bloodstream is reduced. The presence of plaque and damage to the vessel endothelium intensifies the aggregation of thrombocytes and may contribute to the development of thrombotic diseases or infarctions in distant organs.1, 7, 15 Figure 1 shows the pathomechanism of development of atherosclerotic plaque according to Lindhe. Mø+ A Mø Mø B C Foam cells LDL Atherosclerotic plaque D Fig. 1. The pathogenesis of atherosclerosis (by Lindhe, 2003)16 A. Monocytes adhere to the vessel wall. B. Monocytes penetrate into the vessel wall and stimulate the production of cytokines and growth factor. C. The absorption of low density lipoproteins (LDL) causes distension of monocytes and formation of foam cells. D. The wall of the vessel becomes thickened and narrowed due to the proliferation of smooth muscles and development of atherosclerotic plaque. Scannapieco and Genco take several factors into consideration in their explanations of the correlation between atherosclerosis and periodontal diseases. One of them is the direct influence of bacterial pathogens on the development of atherosclerotic plaque; another one is the indirect influence of the inflammatory process on the organism. The genetic predisposition to periodontal diseases and atherosclerosis and the presence of joint risk factors for both units also justify the correlation under discussion.6 The direct influence of infective factors on the vessel wall consists in endotoxins causing damage to the vessel endothelium and initiating a local inflammation. Numerous scientists confirmed the contribution of periopathogens to the formation of atherosclerotic plaque. Porphyromonas gingivalis was found in the atherosclerotic plaque of carotid arteries, aortic and cardiac endothelial cells.15, 17 Furthermore, it was proved that such strains as Porphyromonas gingivalis and Strepptoccocus sanguis may induce thrombocyte aggregation, thus simultaneously favouring thrombotic incidents. The indirect influence of chronic inflammatory processes in the periodontis is also an important mechanism which may account for the dependence under discussion. In the course of inflammatory reaction there is leukocytosis, increased level of C-reactive protein (CRP), fibrinogen and increased concentration of inflammatory mediators: IL-1, TNF-α, sICAM-1, sVCAM-1.8 Moreover, it is known that some bacteria, e.g. Porphyromonas gingivalis have HSP60 proteins, which are identical with human heat shock proteins. This may lead to the initiation of autoimmune reaction. Anti-HSP 65/60 antibodies are produced, which may crossreact with the endothelial antigen and trigger a cascade of atherosclerotic processes.8 Also, the lipopolysaccharides present in the walls of cells of Gram-negative bacteria, e.g. Actinomyces actinomycetemcomitans stimulate the secretion of cytokines, which may damage the vessel endothelium and accelerate cholesterol penetration. In the discussion on genetic predispositions playing an important role in the correlation between periodontal diseases and atherosclerosis Beck found that the presence of the phenotype of Mø (hyperreactive monocytes) is a good reason to include patients into risk groups of atherosclerosis, ischaemic heart disease and periodontitis.1 Offenbacher researched the risk factors of vascular and periodontal diseases. He described the interdependence between atherosclerosis and periodontitis as periodontitis-atherosclerosis syndrome (PAS).18 The author thinks that the highest incidence of the syndrome is in men-smokers, with low social status and suffering from diabetes. In those patients periodontitis has a generalised character with numerous deep periodontal pockets, considerable loss of connective tissue attachment and the presence of a large amount of plaque. He defined the patients qualified for high-risk groups of occurrence of PAS as ‘3:60’, which means that in more than 60% of places in the periodontium the loss of connective tissue attachment makes at least 3 mm. Offenbacher18 also noticed a correlation between the amount of plaque and the diagnosing of periodontal disease in those patients. The more plaque is present on dental surfaces, the higher the risk of occurrence of PAS.18 On the other hand, Beck compared different risk factors of vascular diseases and noticed that periodontitis is a risk factor equivalent to fat consumption, body weight and blood pressure.1 The issue of particular interest is the aforementioned fact that the capability of colonisation of atherosclerotic plaque is attributed to bacteria present in periodontal pockets. In the 1990s molecular biology methods were applied to identify some of the following species of periopathogens in the atherosclerotic plaque structure: Porphyromonas gingivalis, Actinomyces actinomycetemcomitans, Prevotella intermedia, Tanarella forsythiensis, Treponema denticola, Campylobacter rectus, Fusobacterium nucleatum and Eiknella corrodens.15 Numerous publications also confirm the presence of other microorganisms in specimens from the vessels, which do not come from periodontal pockets. They include: Chlamydia pneumoniae, Helicobacter pylori, Mycoplasma pneumoniae or Herpes simplex.18 Periodontal bacteria were found in pathologically changed specimens of vessels in patients with coronary heart disease, carotid artery atherosclerosis, iliac artery atherosclerosis and abdominal aortic atherosclerosis.15 Chiu15 proved the presence of DNA of Porphyromonas gingivalis in 42% and Streptoccocus sanguis in 12% of specimens of the walls of pathologically changed carotid arteries. Mastragelopulos confirmed the presence of bacterial DNA in 59% of the atherosclerotic plaque collected from carotid arteries, whereas Okuda proved the presence of Treponema denticola only in 6 out of 26 specimens collected from the abdominal aorta.19 Ishihara20 noted the presence of Porphyromonas gingivalis, Actinomyces actinomycetemcomitans, Tanarella forsythiensis, Treponema denticola and Campylobacter rectus in the coronary vessels. The bacteria made 21%, 23.3%, 5.9%, 23.5% and 15.0% respectively. The analysis of literature on the subject indicates that not all studies confirm the presence of bacteria related with periodontal diseases in atherosclerotic plaque. In 2005 Fiehn21 in his research based on analysis of specimens collected from carotid and iliac arteries defined the presence of periopathogens as sporadic. In the last decade there have also been studies confirming the correlation between the occurrence of periodontitis and abdominal aortic aneurysms. Aortic aneurysms are defined as a local dilatation of the arterial vessel lumen by 50% as compared with the unchanged segment of the artery located above. They can chiefly be found in elderly patients and are usually related with the abdominal aorta.22 Historical reports on aneurysms from the 19th century associate their occurrence with syphilis. Nowadays it is known that the most significant factor in the process of aneurysm development is the functional and structural loss of elastin from the arterial vessel wall. In consequence of the action of mechanical force the vessel lumen becomes stretched and an aneurysm develops. However, the mechanism that leads to the decomposition of elastin is not known. It has been proved that proteolytic enzymes, such as metalloproteases – elastases, collagenases and gelatinases participate in the process. Furthermore, also other diseases related with connective tissue disorders have been observed in patients with aneurysms. They are: inguinal hernias, emphysema, Marfan syndrome, Ehlers-Danlos syndrome.22 However, there is no unequivocal evidence that atherosclerosis is the direct cause of anuerysms.3 Histopathological examinations of aortic fragments collected from patients with atherosclerosis and from patients with aneurysms do not exhibit many similarities. However, on the basis of latest studies it is known that chronic inflammatory processes participate in the pathogenesis of aneurysms.3 However, the role of microorganisms has not been fully explained yet. The percentage of patients where bacterial cultures confirmed the presence of microorganisms in aneurysm walls fluctuates between 8% and 37%, depending on the research.22 Marques Da Silva 1 proved the presence of such anaerobes as: Propionibacterium acnes, Propionibacterium granulosum, Actinomyces viscosus, Actinomyces naeslundii and Eggertella lenta in 20 out of 28 specimens of aneurysm walls under investigation. Martin et al in their studies based on molecular diagnostics described the case of presence of Actinomyces actinomyctemcomitans from the oral cavity in the aneurysm wall of a patient with chronic periodontitis. Okuda3 found the presence of Treponema denticola only in specimens of abdominal aortic aneurysms. On the basis of a study on 32 patients with abdominal aortic aneurysms Kurihara described the presence of the DNA of Porphyromonas gingivalis in 85% of the patients and Treponema denticola in 63% of the collected specimens. On the other hand, the sporadic occurrence of the DNA of Porphyromonas gingivalis - in 2 cases was proved in PCR examinations of specimens of the walls of aortic aneurysms collected from 22 patients (11 patients with concomitant periodontitis and 11 patients with toothlessness). The presence of four other pathogens under investigation, i.e. Prevotella intermedia, Treponema denticola, Actinobacillus actinomycetemcomitans, Tanarella forsythiensis was not observed in the specimens.23 Summary To sum up the discussion, it is necessary to state that periodontal diseases are one of the most important risk factors related with the inflammatory process in the course of vascular diseases. The analysis of numerous epidemiological, clinical and experimental studies confirms the correlation. However, there still is not full agreement concerning the issue if periodontal bacteria are a factor contributing to the destabilisation of atherosclerotic plaque. It is known that bacteria are a primary factor in the aetiology of periodontal diseases and damaged tissues of the organism are the consequence of immune reactions. Those bacteria stimulate the host cells to secrete enzymes decomposing tissues and to release cytokines activating enzymatic reaction routes. Periodontal diseases cause increased inflammatory response of the organism, which may affect the course of cardiovascular diseases.19 One of the first disorders which may be caused by periopathogens is the dysfunction of vascular endothelium. Inflammatory factors from the periodontis may also influence the development of thrombosis.4 Undoubtedly, both joint risk factors and pathophysiological processes underlie the destabilisation of atherosclerotic plaque and destruction of periodontal tissues. Appropriate periodontological care and treatment of periodontal diseases has influence of reduced risk of occurrence of vascular diseases and patients with vascular diseases should be subject to continuous dental care. References 1.Beck J, Offenbacher S, Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease, J of Periodont 2005, 76, 11-s,2089-2100 2.Beck J, Pankow J, Tylorer H, Offenbacher S, Dental infections and atherosclerosis, American Heart J 1999,Vol. 138,5,528-533 3.Kurihara N, Inoue Y, Iwai T, Umeda M, Huang Y, Ishikawa I, Detection and localization of periodonthopathic bacteria in abdominal aortic aneurysms, Eur J Vasc Endovasc Surg 2004,28,553-558 4.Ziętek M, Periodontal diseases vs atherothromobosis, Czas Stomatol, 2009,62,7,56256 5.Socransky S, Hafajee A., Periodontal microbial ecology, Periodontology 2000, Vol.38,2005,135-187 6.Scannapieco F, Genco R, Association of periodontal infections with atherosclerotic and pulmonary diseases, J Periodont Res 1999, 34, 340-345 7.DeStefano F, Anda R, Kahn H., Williamson D, Russel C, Dental disease and the risk of coronary heart disease and mortality, Br Med J, 1993,306,688-691 8.Konopka T, Periodontal medicine - a new research area in contemporary periodontology, Por Stomat, 2003,8, 38-44 9.Elter J, Champagne C, Offenbacher S, Beck J, Relationship of periodontal disease and tooth loss to prevalence of coronary heart disease. J Periodontol, 2004, 75, 6,782-790 10.Matilla K, Pussinen P, Paju J, Dental infections and cardiovascular diseases: a review, J of Periodont, 2005, 76, 11-s, 2085-2088 11.Syrjänen J., Valtonen V, Ivanainen M, Kaste M, Huttunen J, Preceding infection as an important risk factor for ischaemic brain infraction in young and midlle aged patients, Br Med J, 1988,296,1156 12.Paunio K, Impivaara O, Tiesko J, Maki J: Missing teeth and ischeamic hart disease In men aged 45-64 years. Eur Heart J,1993,14,54-56 13.Kolltveit K, Eriksen H, Is the observed association between periodontitis and atherosclerosis casual?, Eur J Oral Sci 2001,109,2-7 14.Ross R, Atherosclerosis is an inflammatory disease, Am Heart J, 1999,138, S419S420 15.Chiu B, Multiple infections in carotid atherosclerotic plaques, American Heart J 1999, Vol.138, 5,534-536 16.Lindhe J, Karring T, Lang N, Clinical Periodontology and Implant Dentistry 4th ed. Copenhagen:Blackwell/Munsgaaard;2003 17.Deshpande D, Khan M, Genco K, Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis, Infect Immun, 1998, 66,11,5337-5343 18.Offenbacher S, Madianos P, Champagne C, Southerland J, Paquette D, Williams R, Slade G, Beck J, periodontitis- atherosclerosis syndrome: an expanded model of pathogenesis, J Periodont Res, 1999,34,346-352 19.Zaremba M, Górska R, Suwalski P, An assessment of the presence of bacteria related with periodontal diseases in the atherosclerotic plaque of coronary vessels, Czas Stomat, 2005,LVIII,5 20.Ishihara K, Nabuchi A, Ito R, Miyachi K, Kuramitsu H, Okuda K, Correlation between detection rates of periodonhopatic bacterial DNA in carotid coronarystenotic artery plaque and in dental plaque samples, J of Clin Micribiol, 2004, 42, 3, 1313-1315 21.Fiehn N, Larsen T, Christiansen N, Holmstrup P, Schroeder T, Identification of periodontal pathogens in atherosclerotic vessels, J Periodontol 2005, 76, 5, 731-731 22.Marques da Silva R, Lingaas P, Geiran O, Tronstad L, Olsen I, Multiple bacteria in aortic aneurysms, J Vasc Surg. 2003,Vol 38,6,1384-1389 23.Kręgielczak A, Doctoral dissertation “A study on the presence of selected bacterial pathogens in periodontal pockets and specimens of vascular walls in patients with atherosclerosis and abdominal aortic aneurysms” Poznań 2007