Spin polarized transport in semiconductors – Challenges for
advertisement

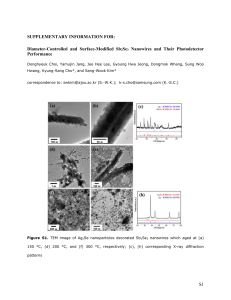
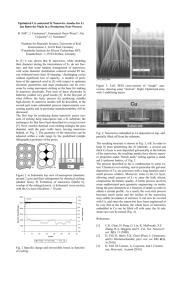
Contribution (Poster) Crystalline Iron Oxide Nanowire Synthesis by Thermal Oxidation C. Morant1, T. Campo1, F. Marquez1,2, J.M. Sanz1, E. Elizalde1 1Departamento de Física Aplicada, C-XII, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain 2IPCAR, Institute for Physical Chemical Applied Research, School of Science and Technology, University of Turabo, 00778PR, USA c.morant@uam.es One-dimensional (1-D) nanostructures, such as nanowires and nanotubes, are the subject of extensive research in optics, magnetism, and electronics due to their enormous potential as building blocks for nanodevices [1,2]. Such 1D materials have properties that differ from those of nanoparticles or materials with larger dimensions. One of the important categories of nanoscale materials are the magnetic ferrites, due to its capability to show unique combination of electronic and magnetic properties. Among those magnetic nanomaterials, magnetite (Fe3O4) and hematite (α-Fe2O3) are of particular interest due to its potential applications, including high-density data storage, biomaterial applications, catalysis, magnetic resonance imaging, sensors, and others. In particular, hematite is one of the most interesting and important metal oxides due to their antiferromagnetic properties and their low toxicity [3, 4]. In this work we report a simple catalyst-free growth method for fabricating highly crystalline iron oxide nanowires by thermal oxidation of iron foils. The growth of iron oxide nanowires has been carried on pure iron substrates in a heated environment (inside a quartz tube furnace). Typical time and temperature range required for the synthesis of a dense nanowire forest (Fig. 1) are two hours and 650ºC-800ºC. The growth properties are studied as a function of temperature, growth time and oxygen partial pressure. The morphology and nanowire density can be controlled by varying growth temperature and time. The morphology, structure and chemical composition of the nanowires obtained were characterized by Field Emission Scanning and Transmission Electron Microscopies (FESEM and TEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). XRD measurements (Fig. 2) indicated that the assynthesized product is in a highly crystalline state of mixed magnetite (Fe 3O4)) and hematite (α-Fe2O3). References [1] G.Cao, D. Liu, Advances in Colloid and Interface Science, 136 (2008) 45-64 [2] W. Lu, C.M Lieber, J. Physics D: Appl. Phys, 39 (2006) R387–R406 [3] A. Nasibulin, S. Rackausas, H. Jiang, Y. Tian, P. R. Mudimela, S.D. Shandakov, L.I. Nasibulina, J. Sainio, E. I. Kauppinen, Nano Res, 2 (2009) 373-379 [4] P. Hiralal, H. E Unalan, K. G. U. Wijayantha, A. Kursumovic, D. Jefferson, J. L. MacManus-Driscoll, G. A. J. Amaratunga, Nanotechnology, 19 (2008) 455608 (7pp) Contribution (Poster) Figure 1 FESEM image of the as-synthesized Fe nanowires Intensity a.u. Figure 2 Nanowires Substrate 20 30 40 50 60 70 80 90 2degrees X-ray diffractogram of the Fe substrate and the iron oxide nanowires grown on the surface as labelled