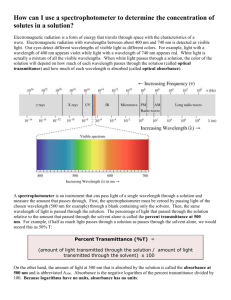
Introduction: Spectrophotometric Analysis is a process through
advertisement

LAB – SPECTROPHOTOMETRIC ANALYSIS Introduction: Spectrophotometric Analysis is a process through which a liquid can be analyzed by shining a light through it. The basic setup of a spectrophotometer is shown below: A cuvette containing the liquid sample is placed between an LED diode lamp and a light detector. The lamp is designed to transmit light at certain specific wavelengths (remember visible light is 400 nm (violet) to 700 nm (red)). The detector can then measure the amount of light that made it through the solution. This can be expressed in one of two manners: * % transmittance: the amount of light transmitted; 0% - 100% a clear solution (such as distilled water) should show a %T of ~100% an opaque solution would show a %T of 0% * absorbance: the amount of light absorbed; 0 – 1.00 a clear solution would show an absorbance of ~0 The absorbance of a solution can be calculated with the following equation: A = abc a – path length b – molar absorptivity c - concentration Purpose: to measure and graph a complete visible spectrum of two or three colored solutions Equipment: Spectrophotometer, test tubes Materials: two or three colored solutions (0.1 M CuSO4 or blue food coloring, 0.1 M NiSO4 or green food coloring, 0.1 M CoCl2 or red food coloring), distilled water Procedure: 1) Turn on the Spectrophotometer to full (left knob), and allow it to warm up for 5-10 minutes while you prepare the solutions. Fill separate test tubes with the solutions provided for analysis. Fill one tube with distilled water to be used for calibration. 2) When the spectrophotometer is properly warmed up, turn the wavelength knob to 400 nm (violet), and look inside the analysis chamber. Can you see the color of the light? Try other wavelengths and record your observations. 3) Set up a data table with 3 columns: wavelength, absorbance, %transmittance. You will be measuring these values from 400-700nm by intervals of 10nm. Create a separate table for each solution you will be analyzing. 4) Measuring Absorbance. Make sure the Spectrophotometer is set to “absorbance”, and the wavelength knob is set to 400 nm. 5) Place the test tube of distilled water in the analysis chamber. Adjust the right knob until the value reads “.00”. Your instrument is now calibrated. 6) Remove the test tube of water, and replace it with the tube containing your colored solution. Record the absorbance at 400 nm. 7) Increase your wavelength by 10 nm, and repeat steps 5-7 until you record the absorbance at 700 nm. 8) Measuring Transmittance. Make sure the Spectrophotometer is set to “%transmittance”, and the wavelength knob is set to 700 nm. 9) Place the test tube of distilled water in the analysis chamber. Adjust the right knob until the value reads “100”. Your instrument is now calibrated. 10) Remove the test tube of water, and replace it with the tube containing your colored solution. Record the %transmittance at 700 nm. 11) Decrease your wavelength by 10 nm, and repeat steps 9-11 until you record the % tranmittance at 400 nm. 12) Repeat steps 4-11 for any other colored solutions provided. LAB – SPECTROPHOTOMETRIC ANALYSIS Data: Recorded on separate paper Analysis: Make 2 graphs, absorbance vs. wavelength, and %transmittance vs. wavelength. If you analyzed more than one solution, superimpose the data on the same graph, making sure to mark clearly which data set belongs to which colored solution. Absorbance vs. Wavelength %Transmittance vs. Wavelength Conclusions: 1) Compare your results of absorbance vs. %transmittance for one single colored solution. How do absorbance and transmittance compare to each other? 2) Compare the absorbances of all the solutions you measured. How do the different colors compare? 3) Spectral analysis is often used to identify unknown substances by comparing an absorbance (or %T) graph to a known standard. Often these spectra are measured in the ultraviolet (UV) or infrared (IR) range. Choose one of your colored solutions, and form a hypothesis describing what you think might happen to its graph if you were able to measure into the IR range (above 700 nm). if there is time, use the spectrophotometer to analyze the solution, and determine the accuracy of your hypothesis