Catalytic Oxidative dehydrogenation
advertisement

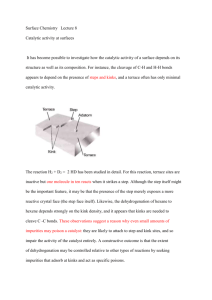
Catalytic Oxidative dehydrogenation The new technology of catalytic oxidative dehydrogenation (ODH) may completely change the way some of the most important organic chemicals are manufactured. The conversion of alkanes like ethane (a by-product of petroleum processing and present in natural gas) to olefins (ethylene, propylene, the butenes, and butadiene) is in great demand in worldwide chemical industry. High operational costs and environmental issues have made this conversion profitable only on a very large scale. With successful development of ODH, high yields of olefins will be possible through the conversion of much smaller volumes of alkanes. Compared with the conventional steam-cracking method of dehydrogenating alkanes to olefins and current catalytic dehydrogenation processes, ODH could reduce costs, lower greenhouse gas emissions, and save energy. Capital and operational efficiencies are gained by eliminating the need for a furnace and for decoking shutdowns, lowering operating temperatures, lessening material demands, conducting fewer maintenance operations, and using a greater proportion of the alkanes in the olefin conversion process. Low molecular weight alkenes, such as ethene and propene, can be formed via non-oxidative dehydrogenation of the corresponding alkane. Non-oxidative dehydrogenation reactions are endothermic and lead to the concurrent formation of carbon and of lower molecular weight alkanes, both of which decrease alkene yields. Oxidative dehydrogenation (ODH) of light alkanes offers a potentially attractive route to alkenes, since the reaction is exothermic and avoids the thermodynamic constraints of non-oxidative routes by forming water as a byproduct. In addition, carbon deposition during ODH is eliminated, leading to stable catalytic activity. However, the yield of alkenes obtained by ODH on most catalysts is limited by alkene combustion to CO and CO2 (COx A relevant example concerns catalytic conversion of n-butane to butenes by oxidative dehydrogenation (ODH) as an alternative process to direct dehydrogenation. It is well known that supported vanadium pentoxide is a promising catalyst for the ODH of n-butane. MgO supported vanadium was reported as a very selective catalyst in the oxidative dehydrogenation of propane and n-butane, while γ-A12O3 supported vanadium catalyst was found to present a good selectivity to olefin products for ethane ODH but a poor selectivity in the ODH of n-butane. The acid-base character of the support explained this different behavior. On MgO, a support with basic properties, the interaction between vanadium species and the supports leads to the formation of vanadate compounds. In the case of more acid supports, such as SiO2 or A12O3 a weak interaction is expected leading to less dispersed vanadium species on the surface which, in turns favors the formation of V2O5 crystallites Catalytic cracking gives many by-products and is not necessarily optimized with respect to propene yield. Propane dehydrogenation, on the other hand, yields propene as the main product. The problem is that the dehydrogenation equilibrium favors propene only at high temperature or low pressure, adding to the overall cost of the process. The need for cryogenic separation of the unconverted propane and produced propene also adds to the process costs. Improvements of the dehydrogenation process to make it commercially more attractive focus on increasing the yield by shifting the equilibrium through removal of one of the reaction products. Thus, in situ removal of hydrogen will shift the dehydrogenation equilibrium to the product side. This can be done either physically by means of a membrane, or chemically by in situ catalytic oxidation using a (post) transition metal or its oxide. The latter approach has the additional advantage of energy release by exothermic oxidation where it is needed to aid the dehydrogenation. However, mixing oxygen, hydrogen, and hydrocarbons at high temperatures creates a highly dangerous mixture. The risk of explosion may be reduced by separating the reactants in space using oxygen-selective membranes.