CHEMISTRY 122 - Seattle Central College
advertisement

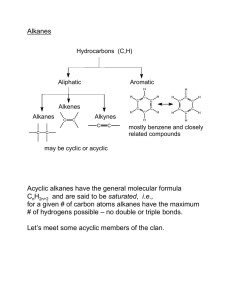
CHEMISTRY 122 CHAPTER #2 ALKANES Sp 2013 14,18 ,19,20,22, 23, 26, 27, 32, 35, 36, 40, 46, 48, 59, 60, 62, 2-14 Line-angle formulas are: (a) (d) (b) (e) (c) (f) 2-18 Structures (a) and (g) represent the same compound. So do structures (d) and (e). Structures (a, g), (d, e), and (f) represent constitutional isomers with molecular formula C4H10O. Structures (b) and (c) represent constitutional isomers with molecular formula C4H8O 2-19 See Appendix A11 2-20 None represent the same compound. There are three sets of constitutional isomers. Compounds (a), (d), and (e) with the molecular formula C4H8O are one set. Compounds (c) and (f) with the molecular formula C5H10O are a second set, and compounds (g) and (h) with the molecular formula C6H10O are a third set. 2-22 The nine constitutional isomers with the molecular formula C7H16 are: 2-23 See Appendix A11 2-26 (a) True (b) False: the octane rating compares the antiknock properties of a test gasoline to that of a reference mixture of 2,2,4-trimethylpentane and heptane, where 100% 2,2,4-trimethylpentane has an octane rating of 100 and 100% heptane has an octane rating of 0. (c) False: they are constitutional isomers, but 2,2,4-trimethylpentane has an octane rating of 100 and octane has an octane rating of –20. 2-27 See Appendix A11 2-32 H H H H H H Staggard (most stable) H H H H H H Eclipsed (least stable) 2-35 See Appendix A11 2-36 No. Cis-trans isomerism results when there is restricted rotation about carboncarbon bonds. Alkanes have free rotation about their carbon-carbon bonds. 2-40 (a) The six-membered rings are in the chair conformation. The five-membered ring is nearly planar, approximating an envelope structure. (b) The hydroxyl on ring A is in the equatorial position. (c) The methyl group between rings A and B is in the axial position. 2-46 Alkanes are nonpolar and insoluble in very polar water. 2-48 Boiling points of unbranched alkanes are related to their surface area, the larger the surface area, the greater the strength of dispersion forces, and the higher the boiling point. The relative increase in size per CH2 group is greatest between CH4 and CH3CH3 and becomes progressively smaller as the molecular weight increases. Therefore, the increase in boiling point per added CH2 group is greatest between CH4 and CH3CH3, and becomes progressively smaller for higher alkanes. 2-59 See Appendix A11 2-60 An octane rating indicates the relative smoothness with which a gasoline blend burns in an automobile engine. The higher the octane rating, the less the engine knocks. The reference hydrocarbons are 2,2,4-trimethylpentane (isooctane), which is assigned an octane rating of 100, and heptane, which is assigned an octane rating of 0. 2-62 2,2,4-Trimethylpentane has a higher heat of combustion both per mole and per gram. 2-65 Molar mass (g/mol) 2,2,4-Trimethylpentane Ethanol 114.2 46.0 Heat of Combustion kcal/mol kcal/g 1304 327 11.4 7.11