Monday “popquiz” on naming compounds
advertisement
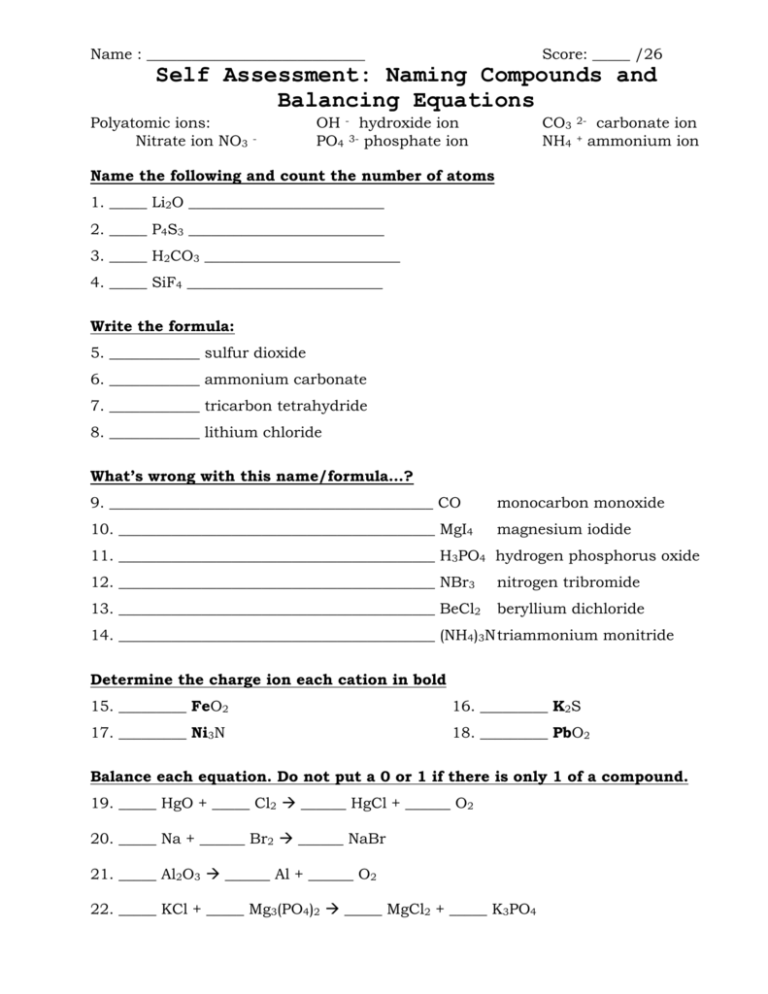
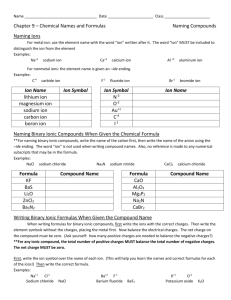
Name : _____________________________ Score: _____ /26 Polyatomic ions: Nitrate ion NO3 CO3 NH4 Self Assessment: Naming Compounds and Balancing Equations - OH - hydroxide ion PO4 3- phosphate ion 2+ carbonate ion ammonium ion Name the following and count the number of atoms 1. _____ Li2O __________________________ 2. _____ P4S3 __________________________ 3. _____ H2CO3 __________________________ 4. _____ SiF4 __________________________ Write the formula: 5. ____________ sulfur dioxide 6. ____________ ammonium carbonate 7. ____________ tricarbon tetrahydride 8. ____________ lithium chloride What’s wrong with this name/formula…? 9. ___________________________________________ CO monocarbon monoxide 10. __________________________________________ MgI4 magnesium iodide 11. __________________________________________ H3PO4 hydrogen phosphorus oxide 12. __________________________________________ NBr3 nitrogen tribromide 13. __________________________________________ BeCl2 beryllium dichloride 14. __________________________________________ (NH4)3N triammonium monitride Determine the charge ion each cation in bold 15. _________ FeO2 16. _________ K2S 17. _________ Ni3N 18. _________ PbO2 Balance each equation. Do not put a 0 or 1 if there is only 1 of a compound. 19. _____ HgO + _____ Cl2 ______ HgCl + ______ O2 20. _____ Na + ______ Br2 ______ NaBr 21. _____ Al2O3 ______ Al + ______ O2 22. _____ KCl + _____ Mg3(PO4)2 _____ MgCl2 + _____ K3PO4 Put it all together. First come up with the compound from each name, then balance the equation. 23. - 24. Potassium iodide and calcium nitrate react to make potassium nitrate and calcium iodide. 25. – 26. Nitrogen gas and hydrogen gas react to form nitrogen trihydride. Answer key_ Name : _ Score: _____ /26 Self Assessment: Naming Compounds and Balancing Equations Polyatomic ions: Nitrate ion NO3 - OH - hydroxide ion PO4 3- phosphate ion CO3 NH4 2+ carbonate ion ammonium ion Name the following and count the number of atoms 3__ Li2O __lithium oxide__ 1. __ 7__ P4S3 __tetraphosphorus trisulfide__ 2. __ 6__ H2CO3 __hydrogen carbonate__ 3. __ 5__ SiF4 __silicon tetrafluoride__ 4. __ Write the formula: SO2__ sulfur dioxide 5. __ (NH4)2CO3_ ammonium carbonate 6. __ C3H4__ tricarbon tetrahydride 7. __ LiCl_ lithium chloride 8. _ What’s wrong with this name/formula…? don’t need mono on carbon_(carbon monoxide)_ CO 9. __ monocarbon monoxide doesn’t balance (Mg2+ I-)_(MgI2)_ MgI4 magnesium iodide polyatomic_(hydrogen phosphate)__ H3PO4 hydrogen 10. __ 11. __ phosphorus oxide nothing…it’s OK______________ NBr3 12. __ 13. __ no “di”, it’s ionic____ BeCl2 nitrogen tribromide beryllium dichloride ionic with polyatomic 14. __ (NH4)3N (ammonium nitride)_____ triammonium monitride Determine the charge ion each cation in bold +4____ FeO2 16. ____ +1___ K2S +1____ Ni3N 18. ____ 15. ____ 17. ____ +4____ PbO2 Balance each equation. Do not put a 0 or 1 if there is only 1 of a compound. 2__ HgO + _____ Cl2 ___2__ HgCl + ______ O2 19. __ 2__ Na + ______ Br2 ___2__ NaBr 20. __ 2__ Al2O3 __4___ Al + ___3__ O2 21. __ 6__ KCl + _____ Mg3(PO4)2 __3__ MgCl2 + __2__ K3PO4 22. __ Put it all together. First come up with the compound from each name, then balance the equation. 23. - 24. Potassium iodide and calcium nitrate react to make potassium nitrate and calcium iodide. KI + Ca(NO3)2 2 KI + KNO3 + Ca(NO3)2 CaI2 2KNO3 + CaI2 25. – 26. Nitrogen gas and hydrogen gas react to form nitrogen trihydride. N2 + H2 NH3 N2 + 3 H2 2 NH3