Recombinant DNA or Pathogenic Infectious
advertisement
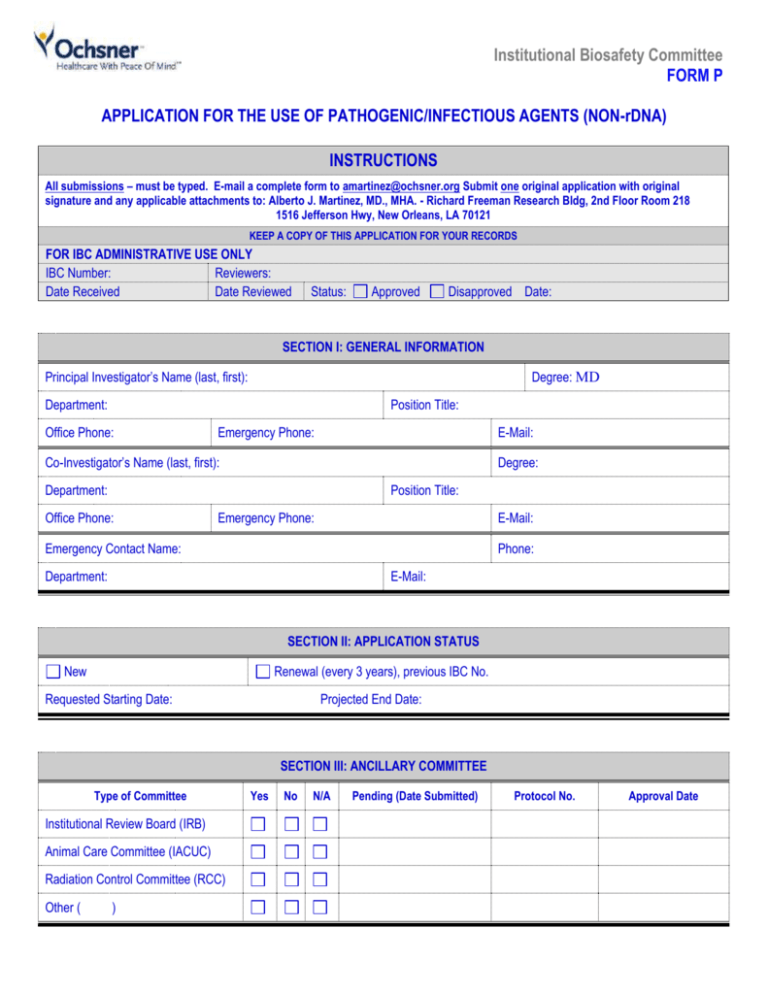
Institutional Biosafety Committee FORM P APPLICATION FOR THE USE OF PATHOGENIC/INFECTIOUS AGENTS (NON-rDNA) INSTRUCTIONS All submissions – must be typed. E-mail a complete form to amartinez@ochsner.org Submit one original application with original signature and any applicable attachments to: Alberto J. Martinez, MD., MHA. - Richard Freeman Research Bldg, 2nd Floor Room 218 1516 Jefferson Hwy, New Orleans, LA 70121 KEEP A COPY OF THIS APPLICATION FOR YOUR RECORDS FOR IBC ADMINISTRATIVE USE ONLY IBC Number: Reviewers: Date Received Date Reviewed Status: Approved Disapproved Date: SECTION I: GENERAL INFORMATION Principal Investigator’s Name (last, first): Degree: MD Department: Position Title: Office Phone: Emergency Phone: E-Mail: Co-Investigator’s Name (last, first): Degree: Department: Position Title: Office Phone: Emergency Phone: E-Mail: Phone: Emergency Contact Name: Department: E-Mail: SECTION II: APPLICATION STATUS New Renewal (every 3 years), previous IBC No. Requested Starting Date: Projected End Date: SECTION III: ANCILLARY COMMITTEE Type of Committee Institutional Review Board (IRB) Animal Care Committee (IACUC) Radiation Control Committee (RCC) Other ( ) Yes No N/A Pending (Date Submitted) Protocol No. Approval Date SECTION IV: RESEARCH SUMMARY Protocol Title: IACUC Protocol Title (If applicable): Funding Source: Please provide a brief summary, including the objectives and experimental design, of the proposed study, emphasizing the nature of any biohazards and procedures for their use, decontamination and disposal. For the benefit of the IBC community representatives, please use non- technical terminology and define all acronyms at first use. Summaries or abstracts from grant applications or other publications should be avoided. (Do not exceed 1 page). SECTION V: LOCATION OF PROJECT Building Room No. Production Storage Experimentation SECTION VI : PATHOGENIC/INFECTIOUS AGENTS 1. Biosafety Level Containment for Proposed Protocol 2. Risk Group Classification for Proposed Protocol BSL1 BSL2 BSL3 RG1 RG2 RG3 3. What infectious microorganism (i.e. causes disease in humans) will be used in this project, and where did you get it? 4. Where will microorganisms be stored? 5. Where will experiments be conducted? 6. Will this experiment involve the infection of animals? Yes No If “Yes”, can infected animal(s) release microorganism into the environment? Yes No 7. Approximately how long after administration could the microorganism be shed? 8. Does individual experiment involve more than one liter of culture? Pathogenic/Infectious Agents (non-rDNA) Revision 7/2010 Yes No Page 2 9. Has staff received initial & annual training in handling the microorganism? Yes No If “Yes” Date 10. Is a vaccine available/recommended for staff handling the microorganism? Yes If “Yes” Please attach documentation of acknowledgement by staff, signed and dated. No 11. Laboratory Biosafety Standard Operating Procedures (SOPs) SOPs are specific description of the potential biological exposure hazards and safety procedures that will be employed to minimize risk. The following issues should be addressed: Safe work practices Disinfection procedures Personal protective equipment Safe transport procedures from room to room or bldg. to bldg. Use of biological safety cabinets Emergency response for exposures Sharps and other waste disposal Emergency response for spills Please describe the building and room number template if you do not have written procedures already in place. where these SOPs will be located. You may use this SECTION VII : LIST ALL PERSONNEL FOR THIS PROJECT Name (last, first) Position Title Pathogenic/Infectious Agents (non-rDNA) Revision 7/2010 Responsibilities Page 3 SECTION VIII : PRINCIPAL INVESTIGATOR’S ASSURANCE 1. I attest that the information contained in the attached application is accurate and complete to the best of my knowledge. 2. I agree to comply with the requirements pertaining to the possession, use, transfer, and disposal of all regulated biologically hazardous materials in accordance to Ochsner policies, applicable federal, state, and local laws and regulations and procedures, including but not limited to OSHA Blood borne Pathogen Standard, the CDC Biosafety in Microbiological and Biomedical Laboratories and NIH Guidelines. 3. I will abide by the reporting requirements and submit a report to the IBC for activities that may include, but not limited to the following: All accident that results in exposure to the infectious agents or recombinant DNA or danger of environmental contamination. All overt spills and spills outside a physical containment equipment (e.g., outside Biosafety cabinet, outside centrifuge, etc). Any problems pertaining to operation, implementation of containment safety procedures or equipment, facility failure, or breach in security (facility and/or biological agent). 4. I understand my responsibility with regard to laboratory safety and certify that the protocol as approved by the IBC will be followed during the period covered by this research project. I certify that no work will be initiated or modified until approval has been issued by the IBC, other appropriate oversight committees and all sponsoring agency requirements have been met. __________________________________________________ Signature of Principal Investigator (must be original) Pathogenic/Infectious Agents (non-rDNA) Revision 7/2010 Date: Page 4










