Ni(II), Cu(II) and Cd(II)
advertisement

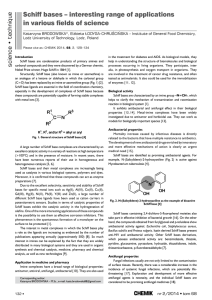
Thermal Behaviour and Thermokinetic Studies of Co(II), Ni(II), Cu(II) and Cd(II) 1,2,4-Triazole-3-Thione Schiff Bases Complexes M.A.El-Gahami, S.A.El-Gyar, A. Abd El-Sameh and S.A.Ibrahim Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt. Email: elgahami@yahoo.com 1,2,4-triazole-3-thiones Schiff bases and their complexes have been reported to be biologically versatile compounds having bactericidal properties(1-3). A series of complexes of 1,2,4-triazole-3-thione Schiff bases with Co(II) Ni(II), Cu(II)and Cd(II) ions have been prepared according to procedure reported in the literature(4). The structure of the complexes was determined by means of elemental analysis, molar conductance, magnetic measurements, infrared and electronic spectral data and thermal studies(4). The thermal decomposition study of Co(II), Ni(II), Cu(II) and Cd(II) complexes was monitored by TG, DTG and DTA analysis in dynamic atmosphere of nitrogen. The thermal degradation takes place in three to five steps depending on the metal ion present. The decomposition course and steps were analyzed and the kinetic parameters: order of the decomposition reaction (n), correlation coefficient (r), activation energy (E) and preexponential factor (collision factor) (Z) of the non-isothermal decomposition steps were computed using Coats-Redfern(5) and Horowitz-Metzger(6) methods and discussed. Comparison of the two sets of kinetic parameters shows that deviation between them is not so great, considering the approximations involved in the Horowitz-Metzger method. Thermodynamic parameters: entropy (S#), enthalpy (H#) and Gibbs free energy (G#) of activation, were calculated using a standard relations(7) and discussed. The dehydration stability of the complexes increases in the order (Cu Ni Co) is in accordance with both Irving-Williams order(8) and the ionic volume of the metal ions(9). The activation energies of the thermal degradation steps lie in the range 0.7-70.61 KJmol-1. Fig.1. TG-DTG curves of [Cu(ABT)2 2H2O]Cl2 (nitrogen atmosphere) Fig.2. Relationships between the initial dehydration temperature or activation energy and metal ion type of ABT complexes Fig3: Horowitz-Metzger plots for the three decomposition steps Cu(ABT)2 2H2O]Cl2 a) first step b) second step c) third step. References 1. Yadawe, M. S.; Patil, S. A. Synthesis, characterization and biological studies of cobalt(II) and nickel(II) complexes with new Schiff bases. Tran. Met. Chem. 1997, 22, 220. 2. Kulkarni, M.V.; Patil, V.D.; Biradar, V.N.; Nanjappa, S. Synthesis and biological properties of some 3-heterocyclic substituted coumarins. Arch. Pharm. 1981, 34 (5), 435. 3. Somorai, T.; Szilagy, G.; Reiter, J.; Bozo, E.; Nagy, G.; Janaky, J.; et al. 1,3,5Trisubstituted 1,2,4-triazole derivatives and medicinal preparation containing them. Eur. Pat. Appl. EP 1985,15548, 81. 4. El-Gyar, A. S.; El-Gahami, A. M.; Abd El-Sameh, A.; Ibrahim, A. S. Synthesis, Thermal decomposition, Magnetic properties and Biological activities of Co(II), Ni(II), Cu(II)and Cd(II) complexes of some Triazole-5-thione Schiff bases. Polish J. Chem. 2007, 81, 1387. 5. Coats, W. A.; RedFern, P. J. Kinetic Parameters from Thermogravimetric Data. Nature, 1964, 201, 68. 6. Horowitz, , H. H., Metzger, G. A New Analysis of Thermogravimetric Traces. Anal. Chem. 1963, 35, 1464. 7. Kandil, S.S.; El-Hefnawy, B. G,; Baker, A. E. Thermal and spectral studies of 5(phenylazo)-2-thiohydantion and 5-(2-hydroxyphenylazo)-2-thiohydantion complexes of cobalt(II), nickel(II) and copper(II). Thermochem. Acta. 2004, 414, 105. 8. Petroni, A. I.; Fertonani, L. F.; Melios, B. C.; Ionashiro, M. Synthesis, characterisation and thermal behaviour of solid state compounds of 4-methylbenzylidenepyruvate with some bivalent metal ions. Thermochem. Acta. 2003, 400, 187. 9. Eugena, A. C. C.; Guedes de Carvalho, R. A.; J. Thermal. Anal. 1971, 2, 269.