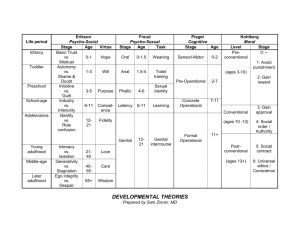
Sexual selection and insect genitalia

Sexual selection and insect genitalia
VOL 1 of 1
Submitted by:
Sahran Higgins
To the University of Exeter as a thesis for the degree of Doctor of Philosophy in Biological
Sciences
June 2009
This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement.
I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University.
………………………………………… (signature)
Abstract
Sexual selection is generally accepted as being responsible for the rapid and divergent evolution of male genitalia and other primary reproductive characteristics in internal fertilisers, such as testes size and sperm length. Selection can act via three main processes: sperm competition, cryptic female choice and sexual conflict, however very few studies have directly addressed the patterns of selection, the degree of phenotypic and genotypic variability expected in genital morphology or the degree to which intromittent genitalia are dependent on male condition. The seedbug, Lygaeus equestris has greatly elongated intromittent genitalia, being almost as long the body. Here I determine whether this is a sexually selected trait and further assess the degree of genetic and phenotypic variability in the greatly elongated male intromittent organ in relation to other morphological components. Further to this, patterns of inheritance and allometry of such exaggerated genitalia were investigated, and of the degree of condition dependence of genital and general morphology was experimentally assessed by varying food availability during ontogeny. Finally, using experimental evolution, I manipulated the level of sexual selection by biasing adult sex-ratio (male-biased, equal-sex, female biased) and investigated potential correlated evolution of female reproductive morphology and fertile (eupyrene) and nonfertile (apyrene) sperm length and numbers in the Indian meal moth, Plodia interpunctella .
The main findings indicated that genital length was sexually selected in L. equestris being negatively related to male fertilisation success and that there was great phenotypic variation in genitalia both across and within populations. Genital length was negatively allometric, in spite of being hugely elongated, and was significantly heritable with considerable evolvability. It was also evident that there was genetic variation in the condition dependence of genital length with a significant genotype-by-condition interaction and much reduced genetic variation in genital length in the poor food treatment. Male and female primary sexual traits of P. interpunctella were also shown to covary, but this pattern did not differ across treatments. Taken together, the results presented in this thesis do not support traditional hypotheses of genital evolution and instead suggests that male intromittent genital length of L. equestris is sexually selected in a similar way to secondary sexual characteristics. This is also true when examining primary sexual traits in P. interpunctella and further highlights the false dichotomy between primary and secondary sexual traits.
Acknowledgements
Most importantly I wish to thank my parents. The convoluted path I have taken to reach this point has been guided by their patience, understanding and unending support of all the decisions have made so far. There is no doubt, that without my parents allowing me to make my own way in life, I would not have achieved half the things I am now so proud of.
You continue to support and encourage me in so many more ways than I can mention.
I would also like to thanks the rest of my family for the years of micky-taking over my choice of career and later my PhD. You definitely helped me keep it real! And also my dear grandparents who saw me begin this journey, but sadly are not here to see me finish.
You provided me with love and encouragement in a way that only grandparents can. I miss you both terribly.
I also thank my supervisors, Dr Nina Wedell and Dr David Hosken, for their patience and guidance during the peaks and troughs of my PhD. The wealth of knowledge they have both (attempted!) to impart on me has always been much appreciated and I am in no doubt that I have benefited hugely from working with two such people. Thanks also to
Dr Dave Shuker and Gethin Evans who allowed me to invade there lab for two months and to Andy Foggo for always having faith in me!
And big thanks to all the people in my life (new and old) who have kept me sane over the last few years with long walks, surfing days and most importantly, long, long sessions in the pub discussing science……. I particularly thank those certain few who were supportive of me in difficult times. Your love and support (Jimmy), voice of reason (Erika,
Kelly D), understanding ear (Ruth), infections desire to party (Michelle, Will, Jez, Kate Cr) and general good laughs, has certainly made for some great memories! Also thanks to my
Worcester ladies, Ruthie and Kate Ca for always refreshing my sense of all things arty in a brain full of science. And finally, to Ted! A great antidote to stress!
This PhD thesis was supported by the Natural Environment Research Council (NERC).
Contents
Title page ..…………………………………………………………………………………1
Abstract.……………………………………………………………………………………2
Acknowledgements...………………………………………………………………………3
Tables and Figures…………………………………………………………………………6
Author’s Declarations…………………………………………………………………….10
1 General Introduction………………………………………………………………….12
1.1 Evolution of insect genitalia …………………………………………………13
1.2 Hypotheses of genital evolution……………………………………………...14
1.2.1 Lock and key………………………………………………………....14
1.2.2 Pleiotropy…………………………………………………………….15
1.2.3 Sexual selection……………………………………………………...16
1.3 Sexual selection and genital evolution……………………………………….18
1.3.1 Intraspecific genital variation………………………………………...18
1.3.2 Genital allometry and variation………………………………………19
1.3.3 Condition-dependence of genitalia…………………………………..22
1.3.4 Genital morphology and fertilisation success………………………..22
1.3.5 Covariation between male-female internal reproductive traits….…...23
1.4 Outline and aims of thesis……………………………………………………24
References…………………………………………………………………..……………...26
2 General methods………………………………………………………………………38
2.1 Study species………………………………………………………………….39
2.2 Lygaeus equestris……………………………………………………………...39
2.2.1 Introduction…………………………………………………………..39
2.2.2 Mating behaviour……………………………………………………..39
2.2.3 Reproductive biology and genital morphology….…………………...40
2.3 Plodia interpunctella…..………………………………………………………42
2.4 Rearing techniques…..………………………………………………………..43
2.5 Matings…..…….………………………………………………………………44
2.6 Sperm counts and measurement..…………………………………………....45
2.7 Body size measurement…….…………………………………………………45
2.8 Genital measurement………...………………………………………………..46
2.8.1 L. equestris
…………………………………………………………...46
2.8.2 P. interpunctella
……………………………………………………...47
References…….……………………………………………………………………………49
3 Intraspecific male genital variation in two populations of the seedbug, Lygaeus
equestris (Heteroptera).………………………………………………………………51
4 Phenotypic and genotypic variation of male genitalia in the seedbug, Lygaeus
equestris (Heteroptera)…………………………………………………………………...72
5 Condition-dependent expression of male genitalia in the seedbug, Lygaeus equestris
(Heteroptera)………………………………………………………………………….87
6
Genital length variation and fertilisation success in the seed bug, Lygaeus equestris
(Heteroptera)………………………………………………………………………...108
7
Experimental evolution of male and female reproductive characters in the Indian meal moth, Plodia interpunctella (Lepidoptera: Pyralidae)………………………121
8 General discussion…………………………………………………………………….143
8.1Conclusion……………………………………………………………………149
References………………………………………………………………………………...150
Tables and figures
Chapter 1
Figure 1: Schematic of positive (a), neutral (b) and negative (c) allometric relationship………………………………………………………………20
Chapter 2
Figure 1: Study species used in chapters 3-6: Lygaeus equestris
(Heteroptera: Lygaeidae) and the Indian meal moth, Plodia interpunctella
(Lepidoptera: Pyralidae) in chapter 7……………………………………40
Figure 2: Genitalia of Lygaeus equestris
: ‘fleshy’ aedeagus (a) bearing a sclerotized processus gonopori (b). Tightly coiled and contained inside the genital capsule on the male abdomen, if intromission is achieved, haemolymph is pumped into the basal end and inflates the aedeagus which then unfolds out of the genital capsule…………………………………...42
Figure 3: Male processus gonopori (a) inside the elastic ductus receptaculi of the female (b). The tip of the processus must penetrate a valve like structure in between the ductus receptaculi and the spermatheca
(sclerotized sperm storage organs) (c)…………………………………...42
Figure 4: Female sperm storage organ (spermatheca) A, and genital tract, B.
Arrows indicate where measurements were taken for each section at the point where the spermatheca constricts in and joins the widest end of the genital tract (scale bar = 1mm). b) Males produce two sperm types; long, nucleate fertile (A) and a smaller, more numerous anucleate non-fertile sperm (B) (scale bar =
100
Figure 5: Light microscope image of the male genitalia and landmarks used in measurement. Aedeagus (A-B) attached to the sclerotized processus gonopori (B-C) (scale bars = 1 mm)……………………………………….47
Figure 6: a) Female sperm storage organ (spermatheca), and genital tract, B.
Arrows indicate where measurements were taken for each section at the point where the spermatheca constructs in and joins the widest end of the genital tract (scale bar = 1mm)…………………………………………….48
Chapter 3
Table 1
. Mean (±SE) body size, life history and male genital length data for males and females of the Sicily and Dolomites populations (untransformed data) (all P ≥ 0.285)………………………………………………………..64
Figure 1: a) Genitalia of Lygaeus equestris : ‘fleshy’ aedeagus (B) bearing a sclerotized processus gonopori (A) (× 75 mag). Circled is the position of the paired structures (b) (arrows) on the sclerotized portion of genitalia of males from the ‘Dolomites’ population, which are absent from males in the
‘Sicily’ population (× 750 mag). (Inset) single genital protrusion (× 1,500 mag.)……………………………………………………………………….65
Figure 2: The relationship between male genital length (mm) and number of eggs fertilized per single male-female pairing (log
10
-log
10
) across the Sicily
(●) and Dolomites (о) populations (n = 31)………………………………..66
Chapter 4
Table 1:
Phenotypic means (±SE) (mm), additive genetic variances (V
A
), coefficient of additive genetic variation (CV
A
), phenotypic coefficients of variation (CV
P
%), allometric dispersion (CV') and narrow sense heritabilities ( h
2
±SE) with associated significance values calculated using
REML……………………………………………………………………...81
Figure 1: Male L. equestris and light microscope image of the male genitalia showing aedeagus (a) attached to the long sclerotized processus gonopori
(b) (scale bars = 1 mm) (x 2.0 magnification)…………………...81
Figure 2: Negative allometric relationship between genital length (a) and male body size and positive association between middle tarsus length and body size
(b)…………………………………………………………………………..82
Chapter 5
Figure 1: Males reared on the bad food diet took an average ~ 4 days longer to reach adult eclosion than those reared in the ‘good’ food treatment (error bars = SE of mean)…………………………………..…99
Figure 2: Genotypic responses of male genital (intromittent organ) length
(mm) in good and bad food quantity environments (GEIs). Each line represents the mean genital length of a split family of full-siblings……100
Chapter 6
Figure 1: Male Lygaeus equestris and light microscope image of the male genitalia and landmarks used in measurement. Aedeagus (a) attached to the sclerotized processus gonopori (B-C) (scale bars = 1 mm)…………………………………………..........................................116
Figure 2: Frequency distribution of male genital length in a population of
L. equestris (a) and (b) Inset b) shows intact (top) and broken (bottom) intromittent organs are easily identified (x 2.0 magnification)…………117
Figure 3: Log-log relationship between intromittent organ length (mm) and number of eggs fertilized per male (average across three females)..118
Figure 4: Log-log relationship between intromittent organ length (mm) and the number of eggs fertilized by the first female……………………… 118
Chapter 7
Figure 1a: Female sperm storage organ (spermatheca) A, and genital tract, B. Arrows indicate where measurements were taken for each section at the point where the spermatheca constricts in and joins the widest end of the genital tract (scale bar = 1mm). b) Males produce two sperm types; long, nucleate fertile (A) and a smaller, more numerous anucleate non-
Figure 2a: Means of eupyrene sperm length across treatments (error bars
= 95% CI) and raw data plots showing the relationships between (b) spermathecae volume and apyrene sperm number, and (d) genital tract length and apyrene sperm number across three selection treatments (ES = equal sex (■), FB = female-biased (▲) and MB = male-biased
( o ))……………………………………………………………………...135
Table 1: Mean (±se) trait differences between male-biased (MB), femalebiased (FB) and equal-sex (ES) ratio treatments……………………….137
Chapter 8
Table 1: Hypotheses for the evolution of male genitalia via sexual selection make a number of predictions concerning the male fitness trait affected by selection, the degree of phenotypic and genetic variation and the level of condition dependence of that trait…………………………144
Author’s declarations
Chapter 1: Introduction: Evolution of insect genitalia
The views presented here are an independent interpretation of the current literature under the guidance of Dr Nina Wedell and Dr David Hosken.
Chapter 2: General methods
The methods presented here are a result of planning and guidance with Dr Nina Wedell, Dr
David Hosken and Dr Zenobia Lewis .
Chapter 3: Intraspecific male genital variation in two populations of the seedbug,
Lygaeus equestris (Heteroptera)
Dr Nina Wedell and Dr David Hosken provided guidance for experimental procedures and preparation of the manuscript. I conducted the experiment, the data collection and data analysis.
Chapter 4: Phenotypic and genotypic variation of male genitalia in the seedbug,
Lygaeus equestris (Heteroptera)
Dr Nina Wedell and Dr David Hosken provided guidance for experimental procedures and preparation of the manuscript. I conducted the experiment at the University of Edinburgh, and collected all data, conducted the analysis and am first author on the manuscript.
Chapter 5: Condition-dependent genital length in the seedbug, Lygaeus equestris
(Heteroptera)
Dr Nina Wedell and Dr David Hosken provided guidance for experimental procedures and preparation of the manuscript. I conducted the experiment at the University of Edinburgh with the help of Gethin Evans, and collected all data and conducted the analysis.
Chapter 6: Genital length variation and fertilisation success in the seed bug, Lygaeus
equestris (Heteroptera)
Dr Nina Wedell and Dr David Hosken provided guidance for experimental procedures and preparation of the manuscript. I conducted the experiment and collected all data and conducted the analysis.
Chapter 7: Experimental evolution of male and female reproductive characters in the
Indian meal moth, Plodia interpunctella (Lepidoptera: Pyralidae)
Dr Nina Wedell, Dr David Hosken provided guidance for experimental procedures and preparation of the manuscript. Dr Zenobia Lewis maintained the selection lines and ran the mating trials and female dissections. I conducted the remaining elements of the experiment, collected the data and conducted the analysis.
Chapter 8: General discussion
The conclusions presented here represent my own interpretation of the previous data chapters under the guidance of Dr Nina Wedell and Dr David Hosken.