Designer Surfactant For Micellar Catalysis
advertisement

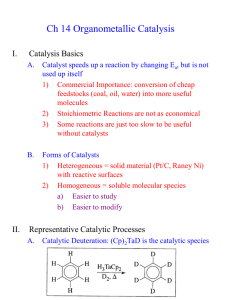
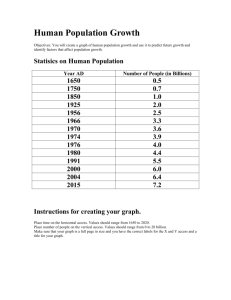
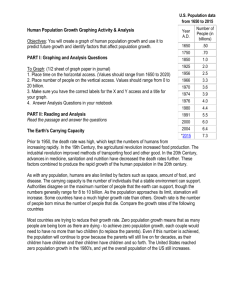
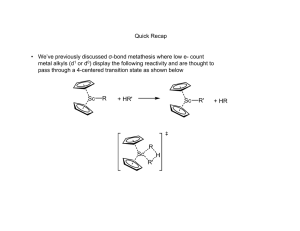
Designer Surfactant For Micellar Catalysis A novel amphiphilic molecule provides a promising platform for carrying out greener ring-closing metathesis reactions in water Stephen K. Ritter A newly designed molecule that functions as a solvent and homogeneous catalyst rolled into one is a promising platform for carrying out greener ring-closing metathesis reactions in water, according to Bruce H. Lipshutz and Subir Ghorai of the University of California, Santa Barbara, who created the amphiphile (Org. Lett. 2009, 11, 705). Polyoxyethanyl ubiquinyl sebacate, or PQS, is made up of a hydroquinone ring that has lipophilic polyprenoid and hydrophilic polyethylene glycol side chains. The ring also has a hydroxyl group that provides a spot for covalently linking a transition-metal catalyst. Given the lipophilic-hydrophilic combination, PQS forms nanoscale micelles in water, which are ideal nanoreactors for ring-closing metathesis reactions, the researchers note. Lipshutz and Ghorai attached a ruthenium carbene catalyst to PQS and tested the micellar system by carrying out a set of reactions using water-insoluble dienes as substrates. They achieved better than 92% yields running the reactions in air at room temperature, even after recycling the system 10 times. PQS provides a "conceptually new, green platform for carrying out micellar catalysis," Lipshutz says.