Supplemental Materials and Methods
advertisement

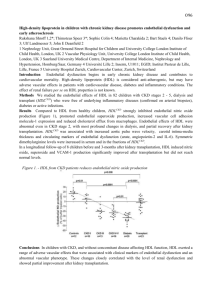
ONLINE APPENDIX Signaling and functional defects of high-density lipoproteins (HDL) in coronary artery disease (CAD) are caused by low sphingosine-1-phosphate (S1P) content and can be efficiently corrected by HDL loading with S1P Katherine Sattler, MD*, Markus Gräler, PhD†, Petra Keul, PhD*, Sarah Weske, MSc*, Christina-Maria Reimann, MSc†, Helena Jindrová, MSc*, Petra Kleinbongard, PhD*, Roger Sabbadini, PhD‡, Martina Bröcker-Preuss, MD§, Erbel Raimund, MD║ (FACC), Gerd Heusch, MD* (FACC), Bodo Levkau, MD* Supplemental Methods Generation of plasma Peripheral venous EDTA blood was drawn from healthy human subjects (n=70) and patients with stable CAD (n=64) recruited consecutively to expand the groups on which our previous findings on CAD-HDLs-S1P were based (1). Venous EDTA mouse blood was obtained by cardiac puncture. Immediately after blood drawing, the tubes were placed on ice. Plasma was generated by centrifugation, immediately recovered and frozen at -80°C. HDL-C in murine plasma was measured with a commercial kit (Fluitest HDL-D, Analyticon Biotechnologies AG, Germany) and by enzymatic methods in human plasma (Advia Chemistry Systems DHDL, CV 2.36%; Bayer Health Care, Germany). The study was approved by the ethics committee of the University Hospital Essen and complies with the Declaration of Helsinki. Written informed consent to participate in the study was obtained from each participant. Demographic and clinical data are provided in Supplemental Table 1. Protein concentration of HDL was determined by Bradford assay and cholesterol with a commercial kit (Fluitest Chol, Analyticon Biotechnologies AG, Germany). Isolation of high-density lipoproteins High-density lipoproteins (HDLs), HDL2 and HDL3 were isolated from pooled plasma (mice) or individual samples (humans) by sequential density gradient ultracentrifugation according to their density (ρ>1.069<1.21g/mL, HDL2: ρ >1.063g/mL<1.125g/mL, HDL3: ρ >1.125g/mL<1.21g/mL (2)) 1 and as already published for these two groups (1). Murine HDLs were isolated from pooled plasma samples of several animals as indicated; human HDLs were isolated from individual plasma samples. Protein concentration of isolated HDL was determined by Bradford assay (BioRad) and total cholesterol concentration with a commercially available kit (Fluitest Chol, Analyticon Biotechnologies AG, Germany), respectively. Oxidation of HDL Isolated HDLs were oxidized with copper sulfate (CuSO4) exactly as described (3). Briefly, HDLs (protein concentration 1mg/mL) were incubated with a freshly prepared CuSO4 solution added to a final concentration of 50μM at 37°C for 250 minutes (4). EDTA was added at a final concentration of 5-fold that of copper (250μM) to stop oxidation. Oxidized HDLs were dialyzed for 24 hours against EDTA-saline (0.5mM EDTA, 150mM NaCl, pH 7.4) in the dark at 4°C with four changes of the dialysis buffer. Optical density of the samples was measured spectrophotometrically at 245nm before and after oxidation and after dialysis to monitor successful oxidation (4). Protein concentrations of oxidized HDL samples were re-determined after dialysis by the Bradford assay. Oxidized HDLs were immediately used for in vitro assays. Measurement of S1P S1P was measured in plasma or lipoproteins by investigators blinded for the experiments as described (1). In brief, lipids were extracted from the samples by successive addition of 1mL of methanol, 200µl of 6mol/L HCl and twice 2mL of chloroform. Chloroform phases were retrieved by centrifugation, and chloroform was removed by vacuum-drying in a speed-vac. Subsequently, samples were dissolved in 100μl of methanol/ chloroform (80:20 v/v). S1P was measured by LCMS. Distribution of S1P in plasma and in HDL was calculated as described (1) . In vitro assays for S1P-loading of isolated HDL Peripheral venous blood of healthy volunteers was collected in vacuum tubes filled with 16IE heparin/mL (Sarstedt). Erythrocytes were isolated by washing the blood sample consecutively with 2 ice-cold phosphate buffered saline (PBS) and erythrocyte buffer (150mmol/L NaCl, 5mmol/L KCl, 21mmol/L Tris-HCl, 0.1% glucose, pH 7.4), and removing in each centrifugation step the white blood cell layer. The cells were diluted to a hematocrit of 0.5 and incubated with sphingosine (final concentration 10μmol/L) for one hour at 37°C. Erythrocytes efficiently generate S1P from sphingosine and do not degrade but release it in the presence of an acceptor (5). After washing the suspension with ice-cold erythrocyte buffer and dilution to a hematocrit of 0.5, HDL or human plasma were added at the indicated concentrations and durations at 37°C under slight shaking. The reaction was terminated by placing it at 4°C, and the supernatants were recovered by centrifugation. In a second approach (direct loading), HDLs were loaded with S1P by adding 0.1 mg of HDL (at a concentration of 1mg/mL HDL protein) to 6 nmol of S1P (for all in vitro studies) or 3 pmol S1P (for all vasodilation studies) after evaporation of its methanol solvent. For this, S1P dissolved in methanol (1mmol/L) was added into Eppendorf cups and placed under a cell culture flow bank for two hours. After the evaporation of the methanol, HDL was added at the indicated concentration and amounts, vigorously vortexed and left at room temperature for one hour. Loading of HDL by this method led to nearly total uptake of the provided S1P as determined by LCMS. Same HDL-S1P values after loading were the same with and without subsequent dialysis demonstrating that HDL stably retained all of the acquired S1P. Injection of C17-S1P–loaded erythrocytes Erythrocytes were isolated from blood of C57Bl/6 mice obtained by cardiac puncture as described above. C17-S1P was added to a final concentration of 10μmol/L to the erythrocyte suspension (hematocrit of 0.5) for 1 hour at 37°C (6). After several washes, 150μl of the suspension were injected into the retroorbital plexus of each mouse. Mice were sacrificed 5 minutes later. Mice Mice heterozygous for HNF 1A (7) were a gift from the Institut Pasteur, Paris. C57Bl/6 mice were from the central animal laboratory of the University Hospital Essen. 4-Deoxypyridoxine (DOP; 6mg/L) was administered with the drinking water for 16 days. Injections were performed on 3 anesthetized animals (isoflurane). The study was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen. Cell culture experiments Chinese hamster ovarian cells (CHO-K1), CHO cells stably transfected with the human S1P1 receptor (CHO-S1P1, both from Novartis) or human umbilical vein endothelial cells (HUVEC) were cultured at 37°C, 5% CO2, in the corresponding media (CHO-K1: F-12 (Ham’s)+GlutaMAX, supplemented with 10% fetal calf serum (FCS), % penicillin/streptomycin/amphotericin B (PSA); CHO-S1P1: aMEM+GlutaMAX, 10% FCS, 1% L-glutamate, 1% PSA, 50μg/ml gentamicin, and 0.5mg/mL G418; HUVEC: RPMI 1640, 20% FCS, 1% PSA, 1% heparin, 0.5% bovine pituitary extract; all cell culture reagents were purchased from Gibco/Invitrogen. Confluent cells were serum-starved overnight (CHO) or for four hours (HUVEC) in serum-free medium prior to incubation with different concentrations of isolated HDL for the indicated durations. The S1P1-receptor antagonists W146 (Avanti Polar Lipids) and NIBr (Novartis), and the S1P3-receptor antagonist TY52156 (8) synthesized and characterized by us previously (9) were used as indicated. In some experiments, HDL-bound S1P was blocked by incubating HDL with different concentrations of Sphingomab (a kind gift of R. Sabbadini, LT1002, Lpath) for 30 minutes at 37°C prior to adding them to the cells. After stimulation, cells were washed once in ice-cold PBS and scraped in lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 5 mM EDTA, 5 mM EGTA, 10 mM NaF, 10% glycerol, 0.5% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaVO4, 1 µg/ml aprotinin, leupeptin, and pepstatin) for 15 min on ice. After centrifugation at 15,000xg for 5 min, the supernatants were taken and protein concentrations determined using the BCA protein assay (Pierce). For functional experiments, a total of HDL preparations from 14 healthy individuals and 9 patients with stable coronary artery disease (sCAD) were randomly chosen from the human HDL sample pool. Demographic and clinical data of these subjects and patients, respectively, are provided in Supplemental Table 2. 4 Immunoblotting Cell lysates (20μg of protein) or HDLs (10μg) were denatured with sample buffer (0.5mol/L TrisHCl, pH 6.8, 10% Glycerol, 10% SDS, 5% b-mercapto-ethanol, 0.1% bromphenol blue) and boiled for 10 minutes. Samples were separated in SDS-polyacrylamide gels and transferred onto Immobilon polyvinylidene difluoride membranes (Millipore). Immunoblotting was performed by incubating the PVDF membranes with primary antibodies against pERK1/2 (1:1000), pAkt (1:500) or peNOS (Ser 1177) (1:100) or the respective total proteins ERK1/2 (1:2000), Akt (1:250) or eNOS (1:100) overnight at 4°C. All antibodies were from Cell Signaling Technologies). Western blotting on HDL samples was performed using antibodies to apoM (Santa Cruz goat polyclonal anti-apoM, 1:200, or Abnova mouse monoclonal anti-apoM, 1:50) or to apoAI (Abcam mouse monoclonal anti-apoAI, 1:1000) for 1 hour at 37°C. Secondary antibodies were HRP-conjugated (against rabbit or mouse, Vector, Santa Cruz) and were used at a dilution of 1:5000 at room temperature for 1 hour. Signals were detected using electrochemiluminescence (Amersham ECL Western Blotting detection reagents, GE) and analyzed/quantified with Image Lab (BioRad). Vasodilation studies Vasodilation studies with HDLs were performed on norepinephrine-precontracted rat mesenteric arteries as previously published (10) and following established protocols (11). Male Lewis rats were sacrificed after enflurane-inhalation anesthesia by rapidly removing the heart in accordance with the German laws for animal welfare and with approval by the local review committee. Segments of 2 mm length of rat mesenteric arteries with intact endothelium were mounted into a Mulvany myograph (Danish Myo Technology, Aarhus, Denmark) and equilibrated with Krebs-Henseleit buffer. Maximal vasoconstriction was defined by the response to potassium chloride (KCl; 1.2*10 -1 mol/L). Subsequently, arteries were washed and re-challenged with norepinephrine (10-5 mol/L) and carbachol (10-4 mol/L) to verify a strong agonist response and endothelial functionality. Endothelial integrity was defined as vasodilation to carbachol by 80% of the norepinephrine-induced preconstriction amplitude. The dilator responses of mesenteric arteries to S1P-loaded or native HDL were determined after preconstriction by 10-5 mol/l norepinephrine. When maximal vasoconstriction had been reached, 5 dilation was induced by adding cumulatively increasing concentrations of native or S1P-loaded HDL. Dilator responses were expressed as percent of the maximum vasoconstriction induced by norepinephrine. Statistics Data are expressed by mean±standard deviation or median (range) for continuous variables, and frequency count and percentage for qualitative variables, respectively. Groups were compared by unpaired or paired t-test, Mann-Whitney-Wilcoxon-U-test or Chi2-test. Vasoconstrictor responses to native and S1P-loaded HDL were compared using 2-way repeated measures ANOVA followed by Bonferroni’s post-hoc tests. P-values are understood to be strictly descriptive. Statistical significance was assumed for P<0.05. Analyses and graphs were performed with Microsoft Office Excel or PASW Statistics 18.0 or 19.0 (Chicago, USA). 6 Supplemental figures — 1 CHO-K1 CHO-S1P1 0.1 0.1 2.5 5 10 — 1 2.5 S1P [µM] 5 10 S1P incubation time [min] pERK1/2 Supplemental Figure 1. Comparison of S1P signaling in CHO-K1 and CHO-S1P1 cells. CHO-K1 control cells and CHO cells overexpressing the human S1P1 receptor (CHO-S1P1) were stimulated with 0.1 µM S1P for the indicated times and ERK1/2 phosphorylation determined by Western blotting. 7 ApoM in HDL/apoM in reference HDL 4.0 P=0.26 3.0 2.0 1.0 0 Controls Supplemental Figure 2. sCAD The HDL content of apoM is similar in healthy and CAD-HDL. The HDL content of apolipoprotein M was determined by Western blotting in HDL (2.5μg of protein) isolated from 70 control subjects and 64 patients with stable coronary artery disease (sCAD). Demographic and clinical data of the subjects are provided in Supplemental Table 1. The HDL sample of one independent, healthy subject was loaded on each SDS-gel to serve as a reference sample. The signal intensity of each apoM band (in arbitrary units) was expressed in relation (ratio) to the reference sample ran on the same gel (in arbitrary units). Densitometry was performed with ImageLab Software (BioRad Laboratory; values for the ratio in healthy HDL (0.86 [0.27-2.49]), values for the ration in CAD-HDL (0.95 [0.223.31]; P=0.26). Box plots show median, minimum and maximum. Circles indicate outliers (values above or below 1.5 times the interquartile range); asterisks indicate extreme values (values above or below 3 times the interquartile range). 8 Supplemental Table 1. Demographic and clinical data of all study participants. Controls Stable CAD (n=70) (n=64) 41 (59) 43 (67) n.s. 51 (20 – 84) 66 (37 – 86) < 0.05 Total cholesterol [mmol/L] 5.68 (3.53 – 7.48) 4.15 (2.79 – 8.93) < 0.05 HDL-Cholesterol [mmol/L] 1.57 (0.75 – 2.94) 1.23 (0.65 – 2.27) < 0.05 LDL-Cholesterol [mmol/L] 3.15 (1.47 – 4.62) 2.24 (1.34 – 5.47) < 0.05 LDL-C:HDL-C ratio 1.96 (0.70 – 3.83) 1.93 (0.83 – 4.16) n.s. n.a. 59 (95) 45 (64) 63 (98) n.a. 18 (28) Male [no. (%)] Age [years] Statins [no. (%)] Hypercholesterolemia [no. (%)] Diabetes mellitus [no. (%)] P-value < 0.05 9 Severity of symptoms [no. (%)] - CCS I n.a. 34 (53) - CCS II 24 (38) - CCS III 6 (9) - CCS IV 0 Extent of disease [no. (%)] - 1-vessel-disease - 2-vessel-disease 17 (27) n.a. 17 (27) - 3-vessel-disease 30 (47) Previous PCI [no. (%)] n.a. 52 (84) Coronary artery bypass graft [no. (%)] n.a. 26 (41) BMI – body mass index, CAD – coronary artery disease, HDL-Cholesterol – high density-lipoprotein-cholesterol, LDL-Cholesterol – low densitylipoprotein-cholesterol, n.a. – not available/not applicable, n.s. – not significant, PCI – percutaneous coronary intervention. Patients were classified as hypercholesterolemic if the diagnosis appeared in the patients’ file regardless of current lipid levels, or if current plasma total cholesterol was > 5.16 mmol/L. CAD patients have lower LDL-C most probably due to their statin medication. Data are presented as mean (minimum – maximum) or as number and percentage. 10 Supplemental Table 2. Demographic and clinical data of all subjects whose HDL were used for functional experiments. Healthy individuals Stable CAD patients (n=14) (n=9) 4 (29) 6 (67) n.s. 35 (20 – 67) 69 (50 – 75) < 0.05 Total cholesterol [mmol/L] 6.24 (3.69 – 6.81) 4.26 (3.46 – 5.44) < 0.05 HDL-Cholesterol [mmol/L] 2.0 (1.06 – 2.37) 1.32 (0.65 – 1.70) < 0.05 LDL-Cholesterol [mmol/L] 3.26 (1.73 – 4.05) 2.13 (1.68 – 3.17) < 0.05 LDL-C:HDL-C ratio 1.6 (0.93 – 2.84) 1.78 (1.06 – 3.15) n.s. n.a. 9 (100) n.a. 7 (70) 9 (100) n.a. n.a. 6 (67) n.a. Male [no. (%)] Age [years] Statins [no. (%)] Hypercholesterolemia [no. (%)] Diabetes mellitus [no. (%)] P-value 11 Severity of symptoms [no. (%)] - CCS I n.a. 5 (56) - CCS II 4 (44) - CCS III 0 - CCS IV 0 Extent of disease [no. (%)] - 1-vessel-disease - 2-vessel-disease 3 (33) n.a. 6 (67) - 3-vessel-disease 0 Previous PCI [no. (%)] n.a. 4 (44) Coronary artery bypass graft [no. (%)] n.a. 3 (33) BMI – body mass index, CAD – coronary artery disease, HDL-Cholesterol – high density-lipoprotein-cholesterol, LDL-Cholesterol – low density-lipoproteincholesterol, n.a. – not available/not applicable, n.s. – not significant, PCI – percutaneous coronary intervention. Patients were classified as having hypercholesterolemia if the diagnosis once appeared in the patients’ file regardless of current lipid levels, or if current plasma total cholesterol was > 5.16 mmol/L. Data are presented as mean (minimum – maximum) or as number and percentage. 12 References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Sattler KJ, Elbasan S, Keul P, et al. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol 2010;105:821-32. Chapman MJ, Goldstein S, Lagrange D, Laplaud PM. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J Lipid Res 1981;22:339-58. Gao X, Jayaraman S, Gursky O. Mild oxidation promotes and advanced oxidation impairs remodeling of human high-density lipoprotein in vitro. J Mol Biol 2008;376:997-1007. Raveh O, Pinchuk I, Schnitzer E, et al. Kinetic analysis of copper-induced peroxidation of HDL, autoaccelerated and tocopherol-mediated peroxidation. Free Radic Biol Med 2000;29:131-146. Bode C, Sensken SC, Peest U, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem 2010;109:1232-43. Sensken SC, Bode C, Nagarajan M, Peest U, Pabst O, Graler MH. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J Immunol 2010;184:4133-42. Shih DQ, Bussen M, Sehayek E, et al. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet 2001;27:375-82. Murakami A, Takasugi H, Ohnuma S, et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Mol Pharmacol 2010;77:704-13. Nussbaum C, Bannenberg S, Keul P, et al. Sphingosine-1-phosphate receptor 3 promotes leukocyte rolling by mobilizing endothelial P-selectin. Nat Commun;6:6416. Nofer JR, van der Giet M, Tolle M, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 2004;113:569-81. Kleinbongard P, Bose D, Baars T, et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res 2011;108:344-52. 13