This exercise is based on concept mapping
advertisement

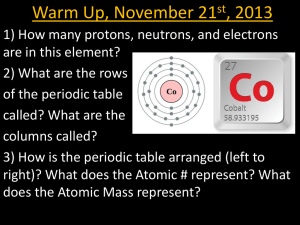
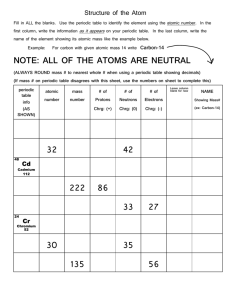
JSSS Teacher Support Material Periodic table - revision This exercise has been adapted from Chemical misconceptions - prevention, diagnosis and cure (ISBN 0-85404-381-0), written by Keith Taber, The Royal Society of Chemistry Fellow 20002001. This book includes many useful classroom activities that can be used to identify student misconceptions, and to help students construct chemical concepts. The exercise below is based on concept mapping. It provides a concept map for the periodic table, with links labelled with numbers. Students are asked to supply sentences to match the links. Below are some suggested sentences that relate to each of the numbers on the diagram. They are not the only sentences that are valid. 1 The periodic table is made up of lots of elements 2 Elements are composed of atoms 3 Each element has a different atomic number 4 Each element in the periodic table is arranged by atomic number 5 Each atom has an atomic number 6 Each element is made up of only one chemical substance 7 A compound is a single substance that can not be broken down easily into its elements 8 Compounds are formed when two or more elements react together 9 An atom has a central nucleus 10 A nucleus contains protons 12 Electrons are the negatively charged particles in an atom 13 The electrons are arranged in shells 14 Not in the picture 15 Get rid of 16 The elements are arranged in rows in the periodic table 17 The rows of elements are called periods 18 The atomic number increases as you go across a period 19 The elements are arranged in columns in the periodic table 20 The columns of elements are called groups 21 Groups of elements usually have similar chemical properties 22 An element’s chemical properties are related to the number of electrons in their outside shell -1-