Chapter 12 4
advertisement

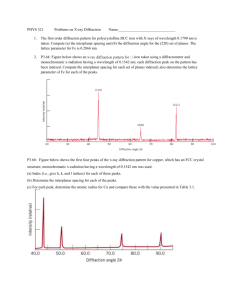
student activity (answers) 12.4 Synchrotron light: X-ray diffraction reading comprehension Answers to student worksheet 1. X-rays of energy 1.32 kiloelectron volts are directed at an unknown crystal. What is the wavelength of the X-rays? [2 marks] E hc / hc /E 6.63 1034 3 108 /(1.32 103 1.6 1019 ) 9.42 1010 m 2. These X-rays diffract from the unknown crystal and the second order, that is when n equals 2 ( n 2 ), and a diffraction bright spot is recorded at an angle 23.4. What is the spacing between the crystal planes? [3 marks] 2d sin n d n /2 sin d 2 9.42 1010 /(2 sin( 23.4)) d 2.37 109 m 3. Why are the X-rays produced in the Australian Synchrotron useful for X-ray diffraction experiments? [1 mark] They can be tuned to a precise wavelength and therefore crystal lattice spacing can be accurately determined. 4. How would the Bragg equation be written for the condition of destructive interference of Xrays? [2 marks] 2d sin n /2 for n=1, 3, 5, 7 … Section 12.4 Page 267 5. a) If we assume that n is of the order of 1, and sin is very close to 1, what can you suggest about the size of the spacing between atoms in a crystal? [1 mark] 2d . Therefore the required wavelength for diffraction is of the order of the size of the crystal lattice spacing. b) Therefore, can you suggest why UV light, visible light, and infrared light are not as useful for diffraction experiments? [1 mark] UV, visible and infrared electromagnetic waves have larger wavelength. Larger that the crystal lattice spacing, and therefore not suitable for the diffraction experiments. 6. If the lattice spacing of a crystal is known, X-ray diffraction can also be used to calculate the wavelength of unknown X-rays. If the first order ( n 1 ) constructive interference location for X-rays is 20°, and the crystal spacing is 0.22 nm, what is the wavelength of the X-rays? [3 marks] 2d sin 1 9 2(0.22 10 )sin( 20) 7. 1.50 1010 m The crystals used in X-ray diffraction often become very hot. Why would this occur? [2 marks] Some of the X-rays would be absorbed and cause electrons to be ionised from the atoms in the crystal. The ionised electrons would have excess kinetic energy and the total internal energy of the crystal would increase when the ionised electrons collide with the atoms in the crystal. Section 12.4 Page 268





![Chapter 12 3 [MS Word Document, 316.5 KB]](http://s3.studylib.net/store/data/007419093_1-fa3113486e018cf1ef8bb10fdbb17649-300x300.png)