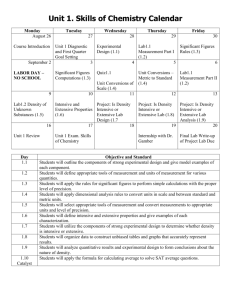
Lab: Physical Properties
advertisement

Physical Properties Name: ____________________________ Date: _____________________ Physical properties are characteristics of a substance that can be observed or measured without changing the composition of the substance. Below is a list of many different physical properties. Use your textbook to find the definitions to these words. (Note: some words may not be in your textbook, feel free to use a dictionary to find any definition that is not in your textbook.) ColorConductivityDensityDuctilityLusterMelting point, Freezing point, Boiling pointMalleabilityMassOdorPhysical StateTasteTemperatureTensile strengthVolatilityVolumeWeight- There are two types of physical properties: Intensive physical properties and Extensive physical properties. Intensive Physical Properties: Properties that do not depend on the amount of the matter present. Extensive physical properties: Properties that do depend on the amount of matter present We will be using clay to help use decide which properties are intensive and which are extensive. Manipulate the ball of clay and decide which properties are dependent on the amount of matter that is present and which one are not. Put the appropriate properties in the box below. Extensive Properties Intensive Properties 1. Can you change any of the intensive physical properties? 2. Can you change any of the extensive physical properties? 3. If you change the mass of the clay, did we change it into a different substance? Explain. 4. If you change the physical state of the clay, did we change it into a different substance? Explain. Changes in physical properties result in a physical change. A physical change in matter is a change that occurs without altering the identity of substances themselves. List three examples of a substance that had undergone a physical change. 1. 2. 3. List three examples of a substance that has been changed but can not be changed back to its original state. 1. 2. 3. What kind of a change is this called?