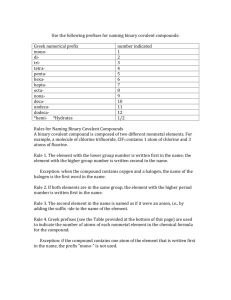
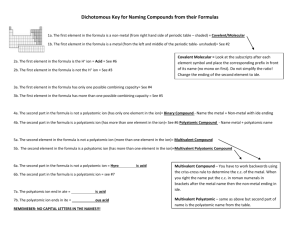
Rules for Naming and Writing Compounds:
advertisement

Rules for Naming and Writing Compounds: I. Standard Nomenclature System 1. Name of the first element + Name of the second element with ending changed to ide. 2. If it is composed of an anion that is a polyatomic ion, write the name of the first element and the name of the polyatomic ion. If the Polyatomic ion is first, write its name and follow with the second element’s name with an ide ending. 3. If the Cation is a metal with more than one oxidation state use the Traditional (Latin Names) or the Stock System (Roman Numerals) to name them and follow with the anion’s name with the ide ending. II. Binary Nomenclature (Covalent Compounds) 1. Use the prefixes mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca, to represent the atoms of each element. 2. The cation should only have a prefix if there is more than one atom present. 3. The anion should ALWAYS have a prefix with the root of the word and an ide ending. 4. Remember that your covalent compounds have been balanced to zero. III. Acids: Naming 1. If the cation of a compound is Hydrogen, then the compound is an Acid. 2. There are two types of Acids: Binary: Consists of Hydrogen and another element, which is a Halide much of the time. To name: Use the prefix Hydro for Hydrogen + the root of the second element with a suffix of ic. Follow with the word Acid. Oxyacids or Tertiary Acids: Hydrogen and a Polyatomic ion. To name: Write the name of the Polyatomic Ion with one of the following endings and follow with the word Acid: If it ends in ate change to ic If it ends in ite change to ous IV. Steps for Determining how to Name Determine if it is an Ionic Compound, Covalent Compound, or an Acid: A) Is it composed of a metal and a nonmetal? Ionic Compound use Standard Nomenclature System Ex. CaS…Calcium Sulfide B) Does the metal have more than one oxidation state? Such as Iron, Mercury, Nickel, etc. …Use standard nomenclature system but use the Traditional or Stock Name for the Metal that identifies which oxidation state is used of that metal. Example FeCl3 Ferric Chloride or Iron III Chloride C) Is it composed of a nonmetal and a nonmetal or a nonmetal and a metalloid? Covalent Compound, use the prefixes to represent molecules with the binary nomenclature. Because they are covalent they are zeroed and you do not have to worry about oxidation states (charges). Example: CO: Carbon monoxide D) Is it composed of a metal and a polyatomic ion or a polyatomic ion and a nonmetal? Use standard nomenclature for this also. NH4Cl Ammonium Chloride K3PO4 Potassium Phosphate E) Is it composed of a cation that is Hydrogen? If so, name as either a Binary Acid or Oxyacid. HCl = Hydrochloric Acid H2SO4 = Sulfuric Acid V. Writing Formulas 1. If the compound is an acid, ionic compound or includes a polyatomic ion either: a) Swap the charges and make them the subscript of the opposite element leaving the + or – out and reduce to the smallest whole number ratio OR b) Balance the compound to zero by determining the least common multiple between the two elements. Example: Sodium Sulfide: Na+1 S-2 => Na2S c) Remember that in order to write these compounds you must know oxidation states (charges) of the elements. 2. If the compound is a binary covalent compound, simply determine the number of atoms of each element based on the prefixes used in the name. Example: Dinitrogen Monoxide N2O