BidusenkoIA_en
advertisement

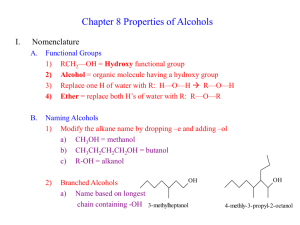
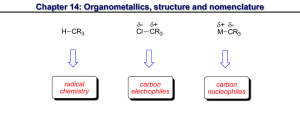
Highly expedient synthesis of propargylic alcohols in the catalytic system Bu4NOH/H2O/DMSO I.A.Bidusenko, N.A.Cherimichkina, E.Yu.Schmidt, B.A.Trofimov A.E. Favorsky Irkutsk institute of chemistry SB RAS, 1 Favorsky Str., 664033, Irkutsk, Russia E-mail: boris_trofimov@irioch.irk.ru Ethynylation of carbonyl compounds is one of the most important reactions of the carboncarbon bonds formation. The reaction affords propargylic alcohols, which represent multifacet intermediates of fine and industrial organic synthesis. However, none of the existing methods for the synthesis of propargylic alcohols is generally applicable to both aliphatic and aromatic carbonyl compounds as well as to monosubstituted acetylenes, and, what is especially important, to acetylene itself (industrially available feedstock). We have developed a new and highly expedient synthesis of secondary and tertiary propargylic alcohols of aliphatic, cycloaliphatic, aromatic and heteroaromatic series. The synthesis is accomplished via organocatalytic ethynylation of aldehydes and ketones with acetylene (under atmospheric pressure) and its monosubstituted derivatives (including functionalized acetylenes) using catalytic composition Bu4NOH/H2O/DMSO.[1] The synthesis opens a facile and environmentally benign (metal-free) way to a plethora of propargylic alcohols, thus essentially expanding the scope of their application as valuable building blocks for the preparation of fragrance compounds, vitamins, pheromones, ecologically friendly insecticides as well as steel acidic corrosion inhibitors and nonionic surfactants. This work was supported Foundation (Project №. 14-13-00588). by a grant of Russian Scientific References [1] E. Yu. Schmidt, N. A. Cherimichkina, I. A. Bidusenko, N. I. Protzuk, B. A. Trofimov Eur. J. Org. Chem. 2014, 4663–4670