Annexure-I GUIDELINES FOR DEALING APPLICATIONS FOR
advertisement

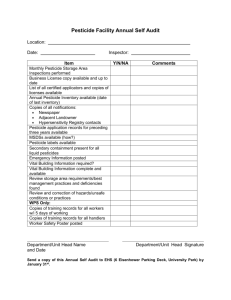
Annexure-I GUIDELINES FOR DEALING APPLICATIONS FOR REGISTRATION UNDER EXPORT ONLY CATEGORY AS APPROVED BY THE RC IN 347TH MEETING I. GENERAL 1. The accreditation of Star Export Houses shall be on the same basis as that of the Ministry of Commerce and the fee shall also be charged accordingly. eapplications would, however, be accepted after development of relevant software. 2. The times lines for export registration under already registered category in case of complete applications shall be on RC-to-RC basis, wherein the applications received till the date of closing of agenda shall be considered in the ensuing meeting. The period for unregistered products shall be three months as the applications are also to be examined from toxicological angle. The period for registration under Star Export House category shall be a fortnight from the date of receipt of applications. 3. The Technical substance(s) and their formulations which have already been approved once by the RC exclusively for exports, these should be included in the proposed Category IV (presently known as Category III) for subsequent registrations exclusively for exports. However, it would commence into force after development of proper database and related software with respect to Certificates of registration and Labels & Leaflets. 4. In case the product is registered for domestic use, a condition in the certificate of registration can be stipulated as “In case of the consignments of the product for export purpose, the primary packaging may be as per the requirement of the importing country”. 5. Deficiencies may be communicated to the applicant through E-mail in case the applicant has provided e-mail ids in Form-I in Cl. 1 under the address as the additional information. 6. Minor corrections, e.g., in label and leaflets etc., may be allowed to be done by authorized company representatives in person on any of the visiting hours on visiting days in the presence of expert concerned. 7. A condition in case of Microbial Biopesticides and Botanical Biopesticides, respectively, shall be incorporated in the certificates of registrations for export category:“The product being exported is in compliance of Biological Diversity Act, 2002.” This condition shall also be applicable in respect of the certificates of registrations already issued. 8. A list of exporters company-wise, product-wise and country export-wise may be maintained in the Sectt. of CIB & RC and the share of Star-Export Houses in total export shall also be maintained future guidance on policy. Necessary amendment in the relevant provision(s) of the Insecticides Rules, 1971 making it mandatory for the registrants to provide export-related information, like the list of exporters company-wise, product-wise and country export-wise shall be made accordingly. 9. A Standing Expert Committee to review/analyse the use and misuse of pesticides in terms of ethics and health, environment, ecology & economy securities shall also be constituted. II. SPECIFIC Guidelines for Registration of Pesticides Exclusively for Export (Refer to Categories at (A) and Requirements at (B) Mentioned Below) (A) Categories of Pesticides for Export Registration Category – I Import of technical grade pesticide, which is not registered for manufacture /Import and use in India, for the sole purpose of Export either as technical itself or after converting it into formulation(s). Category – II Import of technical grade pesticide, which is registered for manufacture/Import and use in India, for the sole purpose of Export either as technical itself or after converting it into formulation(s). Import under this category shall be permitted only from a source approved by the Registration Committee. . Category-III Indigenous manufacture of technical grade pesticide, which is not registered for use in India. Category – IV Indigenous manufacture of pesticide [technical grade with same or different impurity profile or formulation(s) of same, lower or higher concentration with same or different adjuvant including new type of formulation(s)] already registered for use in India (without altering the active ingredients) for the sole purpose of export. Category - V Indigenous manufacture of new pesticide formulation(s), not registered for manufacture and use in the country, for the sole purpose of export. In case of formulation made from technical grade pesticide, which is not registered for use in India, separate application for import/manufacture of technical grade pesticide is also required to be submitted.” Category – VI New combination pesticide formulation, not registered for use in India. In case of unregistered technical grade pesticide(s), separate application(s) is required to be submitted for registration thereof. Category – VII Import of new formulation of pesticide (not registered for import/manufacture and use in India) for the sole purpose of export (In case of formulation made from technical which is registered for use in India, import from approved source of technical only). Category – VIII Indigenous manufacture of registered/unregistered formulation of microbial biopesticides. Category –VIII (a) Bio-pesticides: non-Bt & Bs based Category –VIII (b) Bio-pesticides: Bt & Bs based. Category IX Indigenous manufacture of bio-pesticide--Botanical - technical/ formulation (registered/ unregistered).” (B) Information/Data Requirements for Grant of Registration of Pesticide Exclusively for Export (Refer Table 1 To 5) I. Chemistry Requirements (Category I to IX, Table 1 to 3) :Table 1: (a) Information/data requirements for grant of registration of chemical pesticides exclusively for export (Category I to VII) Sl. No. Parameter 1. 2. 3. Chemical composition Physico-chemical properties Specification in BIS format along with undertaking for product quality Method of analysis Outline of process of manufacture, raw materials used / process of extraction of active ingredient Source of import 4. 5. 6. Category I II III IV V VI VII R R R R R R R R R R R R R R R R R R R R R R NR R NR R R R R R R R R R NR R R NR NR R R R :Table 2: (b) Information/Data requirements for grant of registration of microbial Bio-pesticide exclusively for export (Category VIII) Sl. No. 1. Category – VIII Scientific name, common name, if any, and place of origin in the country. Specify the microbial strain for the given biological activity and its location of existence in India. Composition of the product with percentage of all ingredients. Specification of the product along with undertaking that the product is naturally occurring and not genetically engineered. Outline of process of manufacture, raw material used/process of extraction of active ingredient. Method of analysis R Formulatio n R R R R R R R R R a. Determination of toxin contents using the internationally recognised scientific methods & Standard Operating procedures in accredited and recognised laboratories only for Bacillus sphaericus (B.s) & Bacillus thuringiensis / similar products R R b. Determination of potency by bioassay method R R R R 2. 3. 4. 5. 6. c. OR Determination of Colony Forming Polyhedral Occlusion Bodies(POB) appropriate method Technical Units(CFU)/ counts by Table 3: (c) Information/Data requirements for grant of registration of Botanical Bio-pesticide exclusively for export (Category - IX) Sl. No. Category – IX Technical Formulation 1. Botanical name, common name of the plant and place of origin in the country. Specify the plant part for the given biological activity. Chemical composition and physico-chemical properties. Specification of the product along with the undertaking that the product is naturally occurring and not genetically engineered. Specification in BIS format* along with undertaking for the product quality. Outline of process of manufacture, raw materials used/process of extraction of active ingredient. Method of analysis Determination of active ingredient by appropriate method R R R R R R R R R R R R R R 2. 3. 4. 5. 6. 7. *If no information on certain minor parameters is available the applicant should indicate so and it should be accepted. II. Toxicology Requirements (Category I to IX, Table 4 to 5) Table 4: (a) Toxicology information/data requirements for grant of registration of pesticide exclusively for export [Category I to VII, VIII(a) & IX] Sl. No. Parameter CATEGORY I II II I IV V VI VII ++ + VIII(a) (Microbials) Regd.@ strain/ product 1. 2. 3. 4. 5. 6. IX (Botanicals) NonRegd. Nonregd. Product Regd Strain/ Product product Acute Oral rat Acute dermal R N R R N R R+ ++/R + R NR R NR R R R NR R NR R R+ ++/R + R+ R R N R N R R+ Acute inhalation Primary skin irritation Irritation to mucous membrane Effect on N R N R R NR R NR R R N R R N R R+ R+ R NR R NR R R N R R N R R+ R+ R NR R NR R R N R N N NR NR NR NR NR NR R 7. 8. 9. reproduction Development al Toxicity Carcinogenic ity Mutagenicity R R N R R R N R R N R NR NR NR NR NR NR R N R R N R N R NR NR NR NR NR NR R N R N R R N R N R N R N R NR NR NR NR NR R** R R NR NR NR NR NR NR Neurotoxicit y R R* R R* R* R* R* R* R* R* R* 11. Signs and * * symptoms of poisoning and treatment, first aid and precautionar y measures Published information on these parameters shall be acceptable from any of the following sources: 10. i) ii) From the reviews undertaken by International organization/institutions like WHO, Joint bodies of FAO/WHO, International Registry for Potential Toxic Chemicals (IRPTC), International Agency on Research on Cancer (IARC), etc. Any evaluation / Fact Sheet by Registration authorities of USA or EU or Canada or Japan including Material Safety Data Sheet shall be accepted. Information from other reliable sources including TOXNET, EXTONET can be provided. + If published information is not available data are to be submitted ++ The calculations using WHO formula for conversion of acute LD50 values of technical grade pesticides (which are components of a combination product) into the predicted acute LD50 value of that combination shall be applicable provided the information/data on the technical grade pesticide are available on the same species. +++ Data requirement for category-I additionally will be required. Regd.@ = The product containing the strain registered by Registration Committee. * Applicable only in case of molecules which are not registered for domestic consumption in India. Table 5: (b) Toxicology information/data requirements for grant of registration of Bacillus sphaericus (B.s) & Bacillus thuringiensis (B.t) based pesticide exclusively for export [Category VIII(b)] Sl. No. Toxicity data / information # Category – VIII(b) Bt. & Bs. Based pesticide Technical Formulation 1. 2. 3. 4. 5. 6. 7. Single Exposure Studies Oral toxicity / Pathogenicity Dermal toxicity / Pathogenicity Mucous membrane irritation Primary skin irritation Inhalation toxicity / Pathogenicity Allergy / Sensitization Sign & Symptoms of poisoning/ First Aid, treatment & other precautionary measures R R R R R R R* R R R R R R R* Note : Allergy test has to be conducted as per Standard Protocol prescribed by Registration Committee. # If a product is already registered strain then there is no necessity of above information. * Applicable only in case of molecules which are not registered for domestic consumption in India. III. Packaging and Labelling Requirements for All Categories All Categories (I to IX) 1. 2. An undertaking with respect to type of packaging. Labels and Leaflets as per Insecticides Rules, 1971.