Molar Concentration
advertisement
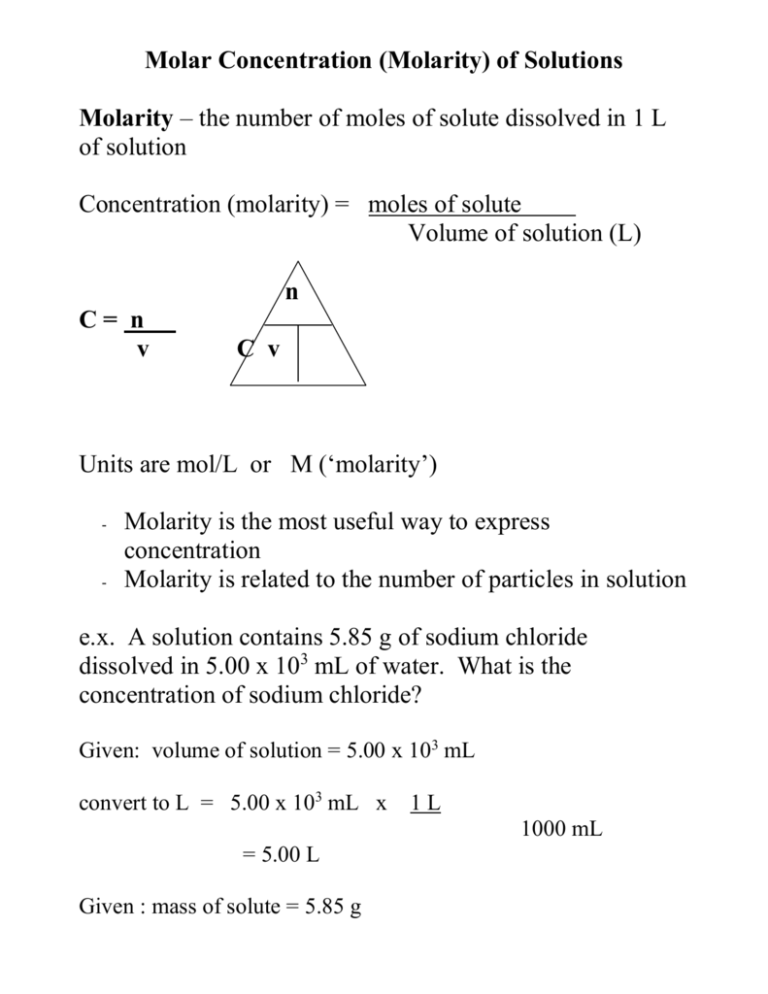
Molar Concentration (Molarity) of Solutions Molarity – the number of moles of solute dissolved in 1 L of solution Concentration (molarity) = moles of solute Volume of solution (L) n C= n v C v Units are mol/L or M (‘molarity’) - - Molarity is the most useful way to express concentration Molarity is related to the number of particles in solution e.x. A solution contains 5.85 g of sodium chloride dissolved in 5.00 x 103 mL of water. What is the concentration of sodium chloride? Given: volume of solution = 5.00 x 103 mL convert to L = 5.00 x 103 mL x 1L 1000 mL = 5.00 L Given : mass of solute = 5.85 g Find: Concentration in mol/L So we must first find # of moles of solute Use n = m M (# moles = mass of sample ) molar mass Molar mass NaCl = 22.99 + 35.45 = 58.44 g/mol n=m Mx n= 5.85 g 58.44 g/mol n = 0.1001 mol Now we can plug this into our molarity equation.. C= n C = 0.1001 mol 5.00 L V C = 0.0200 mol/L Therefore, [NaCl] in the solution is 0.0200 mol/L e.x. A solution contains 6.00 g of sodium phosphate dissolved in 250.0 mL of distilled water. a) Find the concentration of sodium phosphate b) Find the concentration of sodium ions and phosphate ions a) Na3PO4 Molar mass = 3(22.99) + 30.99 + 4(16.00) = 163.94 g/mol n= m M n = 6.00 g 163.94 g/mol Find [Na3PO4]: n = 0.036599 mol C= n v C = 0.036599 mol 0.250 L C = 0.146 mol/L b) Find concentration of sodium and phosphate ions. Na3PO4 3 Na+ [Na+] = 3 [Na3PO4] = 3 (0.146 mol/L) = 0.439 mol/L + PO4 3[PO4 3-] = [Na3PO4] = 0.146 mol/L Molar Concentration (Molarity) of Solutions Molarity – the number of moles of solute dissolved in 1 L of solution Concentration (molarity) = moles of solute Volume of solution (L) n C= n v C v Units are mol/L or M (‘molarity’) - Molarity is the most useful way to express concentration Molarity is related to the number of particles in solution e.x. A student dissolves 5.85 g of sodium chloride in water to make a solution of 5.00 x 103 mL. What is the concentration of the solution in mol/L e.x. A 250.0 mL solution contains 6.00 g of sodium phosphate dissolved in distilled water. a) Find the concentration of sodium phosphate in the solution b) Find the concentration of sodium ions and phosphate ions Read pages 313 – 316 # 19 -24 pg 316







