8_Chemical Bonding and Molecular Shapes
advertisement
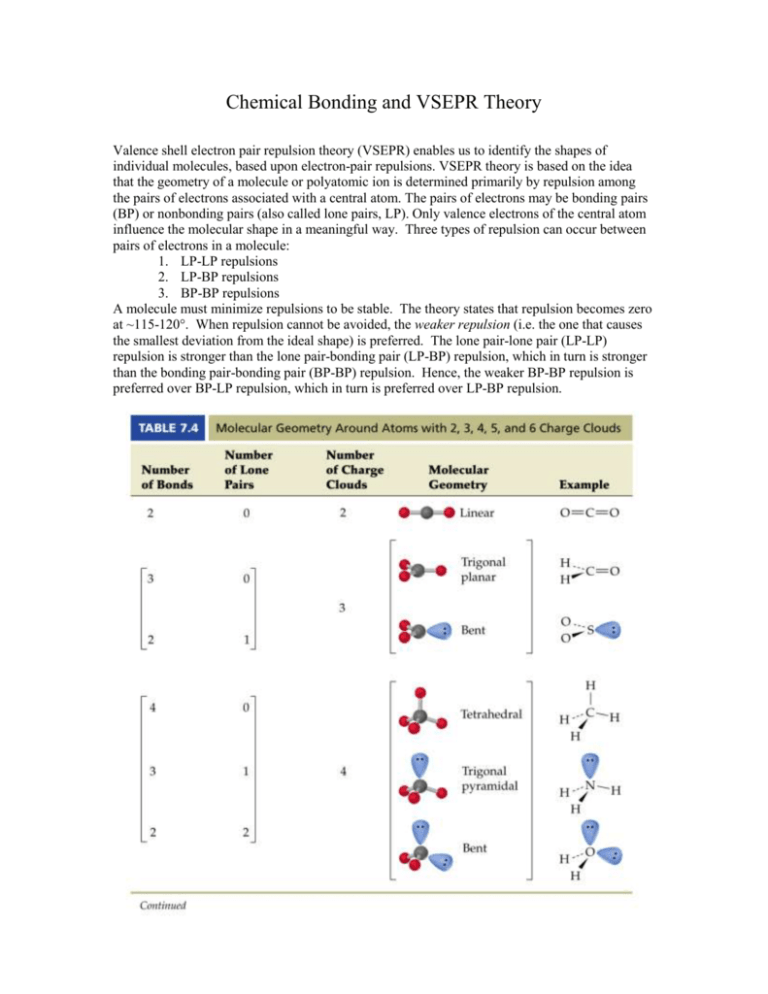
Chemical Bonding and VSEPR Theory Valence shell electron pair repulsion theory (VSEPR) enables us to identify the shapes of individual molecules, based upon electron-pair repulsions. VSEPR theory is based on the idea that the geometry of a molecule or polyatomic ion is determined primarily by repulsion among the pairs of electrons associated with a central atom. The pairs of electrons may be bonding pairs (BP) or nonbonding pairs (also called lone pairs, LP). Only valence electrons of the central atom influence the molecular shape in a meaningful way. Three types of repulsion can occur between pairs of electrons in a molecule: 1. LP-LP repulsions 2. LP-BP repulsions 3. BP-BP repulsions A molecule must minimize repulsions to be stable. The theory states that repulsion becomes zero at ~115-120°. When repulsion cannot be avoided, the weaker repulsion (i.e. the one that causes the smallest deviation from the ideal shape) is preferred. The lone pair-lone pair (LP-LP) repulsion is stronger than the lone pair-bonding pair (LP-BP) repulsion, which in turn is stronger than the bonding pair-bonding pair (BP-BP) repulsion. Hence, the weaker BP-BP repulsion is preferred over BP-LP repulsion, which in turn is preferred over LP-BP repulsion. The figure above illustrates trigonal planar (3 balloons), tetrahedral (4 balloons), trigonal bipyramid (5 balloons), and octahedral (6 balloons) geometries. The balloons represent electron domains (LP or BP). Example trigonal planar geometry Example tetrahedral geometry The ideal geometry associated with 4 electron domains is that of a tetrahedron. If all four electron domains involve bonding pairs (BP), that is, if the central atom is bonded to four other atoms, the molecular shape is tetrahedral. If the tetrahedron includes one LP (NH3), the molecular shape is that of a trigonal pyramid. The LP compresses the ideal bond angle down from 109.5˚ to 107˚. If the tetrahedron includes two LPs (H2O), the molecular shape is angled or bent. The LP compresses the ideal bond angle down from 109.5˚ to 104.5˚. Phosphorus pentachloride, PCl5, possesses a trigonal bipyramid shape. What molecular geometry results in SF4 (4 BP, 1 LP), ClF3 (3 BP, 2 LP), or I3-1 (2 BP, 3 LP)? Sulfur hexfluoride, SF6, possesses an octahedral geometry. What molecular geometry results in SbCl5-2 (5 BP, 1 LP), or XeF4 (4 BP, 2 LP)? Chemical Bonding and Valence Bond Theory Valence bond theory features the overlapping of atomic orbitals when atoms form a chemical bond. Atomic orbitals from different atoms may overlap in two distinct ways, producing sigma bonds () and pi () bonds. Sigma bonds, the stronger of the two types of bonding, occur when two half-filled orbitals overlap head-on along the internuclear line of the bonded atoms. Pi bonds occur when two orbitals overlap sideways. For example, a bond between two s-orbital electrons is a sigma bond, because two spheres are always coaxial. In terms of bond order, single bonds have one bond, double bonds consist of one bond and one bond, and triple bonds contain one bond and two bonds. Head-on overlap - a sigma () bond Hybridized Orbitals The basic idea of valence bond theory is that a covalent bond is formed by the overlap of atomic orbitals. The two electrons of paired spin, which are shared by two bonded atoms, lie in an atomic orbital of each of the two atoms. The greater the degree of overlap of the atomic orbitals, the greater will be the degree of sharing and the stronger will be the covalent bond between them. Often, however, the geometry of these orbitals is such that effective overlap cannot occur in the known geometry of the molecule. Under these circumstances, the atomic orbitals on an atom can reconfigure themselves into a different configuration, and the reconfigured orbitals are said to be hybridized. Hybridization of atomic orbitals was first proposed by Linus Pauling. The hybridization (mixing) process affects the spatial arrangement and energies of the hybrid orbitals. Hybrid orbitals are simple to envision, and they can be used to explain the observed shapes and bond orders for p-block molecules. They also make the distinction between sigma and pi bonding easy to understand. Methane, CH4, makes use of sp3 hybridization, producing a tetrahedral molecule. Ethylene, C2H4, makes use of sp2 hybridization in both carbon atoms. The sp2 hybrid orbitals on each carbon atom point towards the corners of an equilateral triangle. The carbon atoms are bonded together by the sigma-overlap of two sp2 hybridized orbitals. The remaining two sp2 hybrid orbital on each carbon overlap the half-filled 1s atomic orbitals of two hydrogen atoms. The remaining unhybridized p-orbital on one carbon atom overlaps sideways with the unhybridized p-orbital on the other carbon atom, producing a bond. Acetylene, C2H2, employs sp-hybridization in its carbon atoms. The sp hybrid orbitals on each carbon atom point in opposite directions. The carbon atoms are bonded together by the sigma-overlap of two sp hybridized orbitals. The remaining sp hybrid orbital on each carbon overlaps a half-filled 1s atomic orbital of a hydrogen atom. The pair of unhybridized p-orbitals on one carbon atom overlap sideways with those on the other carbon atom, producing a pair of bonds. Explaining Trigonal Bipyramidal and Octahedral Molecular Geometries Trigonal bipyramidal geometry is explained by hybridizing an s-orbital with three porbitals and a d-orbital. This is known as sp3d hybridization. Octahedral geometry is explained by hybridizing an s-orbital with three p-orbitals and two d-orbitals. This is known as sp3d2 hybridization. This project is funded by a grant awarded under the President’s Community Based Job Training Grant as implemented by the U.S. Department of Labor’s Employment and Training Administration (CB-15-162-06-60). NCC is an equal opportunity employer and does not discriminate on the following basis: against any individual in the United States, on the basis of race, color, religion, sex, national origin, age disability, political affiliation or belief; and against any beneficiary of programs financially assisted under Title I of the Workforce Investment Act of 1998 (WIA), on the basis of the beneficiary’s citizenship/status as a lawfully admitted immigrant authorized to work in the United States, or his or her participation in any WIA Title I-financially assisted program or activity. This product was funded by a grant awarded under the President’s High Growth Job Training Initiative, as implemented by the U.S. Department of Labor’s Employment & Training Administration. The information contained in this product was created by a grantee organization and does not necessarily reflect the official position of the U.S. Department of Labor. All references to non-governmental companies or organizations, their services, products, or resources are offered for informational purposes and should not be construed as an endorsement by the Department of Labor. This product is copyrighted by the institution that created it and is intended for individual organizational, non-commercial use only.








