solvents atom
advertisement

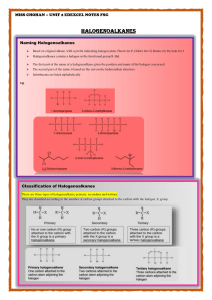
CI 13.1 Halogenoalkanes Are man-made compounds with one or more halogen atoms (F, Cl, Br, I) attached to a carbon atom. The attached halogen changes the chemical properties of alkane chains…they are very unreactive, and so have been very useful to humans. Naming halogenoalkanes (haloalkanes) (similar rules to naming alcohols, just add the halogen as a prefix): halogens are in alphabetical order. lowest numbers possible are used. CH3CH2CH2Cl is 1-chloropropane CH3CHClCH2Cl is 1,2-dichloropropane CH3CHBrCH2CH2Cl is 3-bromo, 1-chlorobutane. CH3CHICHBrCH2Cl 2-bromo,1-chloro,3-iodobutane 2-bromo, 3-chloro, 1-iodopentane CH2ICHBrCHClCH2CH3 Physical properties of halogenoalkanes immiscible with water The bigger the halogen atom /the larger the number of halogen atoms the higher the boiling point. Larger halogen atoms (Br or Cl) cause greater environmental damage than smaller halogen atoms (F); this is important when designing replacements for CFCs. Chemical reactions of halogenoalkanes Carbon –halogen (C-Hal) bonds can break either homolytically or heterolytically. Homolytic Fission forms radicals eg when a halogenoalkane absorbs radiation of the right frequency. H H H C Cl + hv H H C + Cl H Chloromethane methyl radical Shorthand is: CH3-Cl + hv CH3 + Cl chlorine radical (occurs in stratosphere). Heterolytic fission is more common in lab conditions using polar solvents such as ethanol or ethanol and water. The polar C-Hal bond can break, leaving a negative halide ion and positive carbocation. CH3 CH3 C CH3 Cl + hv H CH3 C+ + ClH 2-chloro-2-methylpropane carbocation chloride ion (negatively charged substances may react with the positive carbocation causing a substitution reaction). Importance of reaction conditions…for determining how bonds break Eg. Bromoethane C-Br bonds break: Heterolytically, forming ions when dissolved in a polar solvent (say a mixture of ethanol and water) BUT Homolytically, in the gas phase at high temp. or when dissolved in a non-polar solvent, such as hexane. Different halogens, different reactivity. All reactions with halogenoalkanes involve breaking the C-Hal bond. The C-F bond is the strongest (bond enthalpy 467 kJmol-1) and therefore the hardest to break, whereas the C-I bond is relatively weaker (228 kJmol-1) and therefore easier to break. C-Hal bonds get weaker, and so more reactive, down group 7. Chloro compounds are fairly unreactive and remain in the troposphere long enough to reach the stratosphere, where they react with and destroy the ozone layer. Substitution reactions of halogenoalkanes Halogenoalkanes can hydrolysed by hydroxide ions to form alcohols. Eg. Bromobutane forms butanol: CH3–CH2–CH2–CH2–Br + OH- CH3–CH2–CH2–CH2–OH + BrThe C-Br bond is polar C–Br The oxygen atom on OH- is –vely charged. H–O The partial positive charge on the carbon atom attracts the negatively charges oxygen of the hydroxide ion. A lone pair of electrons on the O atom forms a bond with the C atom as the C__Br bond breaks. H H H H H C__C__C__C__Br H H H H H H H H H C__C__C__C__O__H H H H H __ __ _ O H _ + Br Heterolytic fission results in IONS and not radicals. Curly arrows show the movement of electrons (full headed arrows for a pair of electrons…unlike radical reactions). Halogenoalkanes can give substitution reactions with hydroxide ions and other NUCLEOPHILES. Nucleophiles can donate a pair of electrons to a positively charged carbon atom to create new covalent bonds. Some common nucleophiles: Name Formula Hydroxide ion OH- Structure showing lone pairs _ H__O CH3COO- Ethanoate ion _ __ __ CH3 C O O C2H5O- Ethoxide ion _ __ CH3CH2 O Water molecule H2O O H Ammonia molecule H NH3 N H H H CN- Cyanide ion _ N C The carbon atom attacked by the nucleophile may be part of a carbocation and carry a full positive charge, or it may be part of a neutral molecule (as in the above example with bromobutane) and carry a partial positive charge as a result of bond polarisation. If X- represents a nucleophile, the nucleophilic substitution process is: X C–Hal __ C X + Hal Water as a nucleophile Nucleophiles may be neutral or have negative charge, so long as it has a lone pair of electrons which can form a bond to a carbon atom. Eg. Water has 2 lone pairs of electrons on the O atom. First it attacks the halogenoalkane (bromobutane in this case): H H H H H C__C__C__C__Br H H H H H H H H H C__C__C__C__O__H H H H H H __ __ _ + Br + H+ O H H The resulting ion loses H+ to form an alcohol: H H H H + __ __ __ __ H C C C C O H H H H H __ H H H H H C__C__C__C__O__H H H H H __ The overall equation for the reaction of water with a genera; halogenoalkane R__Hal is: R__Hal + H2O + R__OH + H+ + Hal- Ammonia as a nucleophile A lone pair of electrons on the N (similar to water) attacks the halogenoalkane to produce an AMINE with an NH2 group: R__Hal + NH3 R__NH3+ Hal- R__NH2 + H+ + Hal- Using nucleophilic substitution to make halogenoalkanes Halogenoalkane + OH- alcohol Halogenoalkanes can be made via the reverse reaction of making alcohols; the nucleophile is Hal-. Eg. 1-bromobutane is made using a nucleophilic substitution reaction between butan-1-ol and Br- ions, in the presence of a strong acid. Ist step: H+ ions bond to O atom on the alcohol: H H H H H H H H + __ __ __ __ __ __ __ __ __ __ H C C C C O H C C C C O H H H H H H H H H H H+ This gives the C atom to which the O is attached a greater partial positive charge. It is now more readily attacked by Br- ions, forming bromobutane. __ H H H H + H__C__C__C__C__O H H H H H _ Br H H H H H__C__C__C__C__Br H H H H + H 2O The overall equation for the reaction is: CH3CH2CH2CH2OH + H+ + Br- CH3CH2CH2CH2Br + H2O Activity A4.2 Problems for 13.1 pages 303- 304 questions 1- 9.