Chemistry 12 Course Outline
advertisement

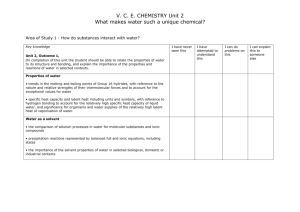
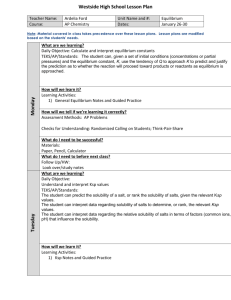
Chemistry 12 Course Outline September 2015 – January 2016 Instructor: Ms. Crystal Gaudry (crystal.gaudry@sd71.bc.ca) Overview: Chemistry is the study of composition, properties and interactions of matter. Chemical knowledge advances within a social context and it is important for students to realize that the principles and laws they accept as fact are the result of extensive observations and analysis done by scientists. Chemistry is the study of matter and its changes. Through the study of chemistr y, the learners are given an opportunity to explore and understand the natural world and to become aware of the profound influence of chemistry in their lives. The major goals. of Chemistry 12 are: • To develop in students an understanding of the big interconnecting ideas and principles that transcend and unify the natural science disciplines. • To provide students with an enhanced world view, inquiry and enterprise. • To help students attain the level of scientific awareness essential for all citizens in a scientifically literate society. • To help students make informed decisions about further studies and careers in science. • To provide students with opportunities for acquiring knowledge, skills and attitudes that contribute to personal development. Units of Study: 1. Brief Review of Chemistry 11 This will include nomenclature, balancing equations, and stoichiometry. 2. Reaction Kinetics Explain the significance of reaction rates, demonstrate how rates can be measured and explain, with reference to collision theory and reaction mechanisms, how rates are altered. Important Concepts: Reaction rates; Collision theory; Activation, Kinetic and Potential Energy; Reaction Mechanisms; Catalysts. 3. Dynamic Equilibrium Analysis of reversible reacting systems, with reference to equilibrium systems, Le Châtelier’s Principle, and the concept of a reaction constant, K eq. Important Concepts: Reversibility of a reaction and Dynamic Equilibrium; Le Chatelier’s Principle; Equilibrium Concentrations and calculations;Equilibrium constants (Keq); Effects of Temperature and Pressure. 4. Solubility Equilibrium Explain solute-solvent interactions in solubility equilibrium and describe the significance of Ksp with respect to saturated systems. Concepts include ionic vs. molecular solutions, relative solubility of solutes, solubility rules, and equilibrium in saturated solutions. Important Concepts: Ionic vs Molecular solutions; Relative solubility of solutes; Solubility rules; Solubility product calculations (Ksp); Common ion effect; Equilibrium in Saturated solutions. 5. Acids and Bases Nature of Acids & Bases: Describe the specific characteristics of acids and bases and distinguish the varying strengths of acids or bases for equilibria using a Brönsted-Lowry model. Quantitative Problem Solving: Describe the special role played by water in aqueous systems and use the acid-base equilibrium constants (Ka and Kb) and the ionization constant of water (Kw) to calculate pH and pOH values for different acid-base equilibria. Applications of Acid-Base Reactions: Identify practical applications of acid-base systems, demonstrate the use of titrations to determine quantities of materials, explain the significance of hydrolysis, and relate buffer systems and acid rain to the concept of acid-base equilibrium. Important Concepts: Arrhenius and Bronsted-Lowry; Conjugate Acid-Base pairs; Strong and Weak acids & bases; Amphiprotic; pH/pOH, Kw, Ka & Kb calculations; Indicators; Titrations; Hydrolysis; Applications. 6. Oxidation-Reduction Describe the essential components of reacting systems that involve electron transfer, determine the stoichiometry of redox reactions by balancing redox reactions, and apply their findings to perform redox titrations. Applications of Redox Reactions: Use the concept of spontaneous and non-spontaneous reactions to explain practical applications of redox such as batteries, electroplating, electrorefining, and corrosion. Important Concepts: Definitions; Oxidation numbers; Balancing Half-Reactions & Redox Reactions; Standard Reduction Potentials; Predicting spontaneity; Electrochemical Cells; Electrolytic Cells. Evaluation: Exams/Quizzes Assignments & Labs 70% 30% * You are responsible for all assigned work. Homework is to be done prior to class. ** There is NO Provincial Exam for this course. You will write a Final Exam at the completion of the course worth 25% of your final grade. Required Textbooks: Hebden Chemistry 12: A Workbook for Students Heath Chemistry Technology: There is a class page located on the Vanier web site. Expectations: Be prepared for class with all the materials you will need (including your textbook and notes) Be prepared to work Be punctual Be respectful of yourself and others Be here – attitude and attendance are critical to success No cell phones in class please