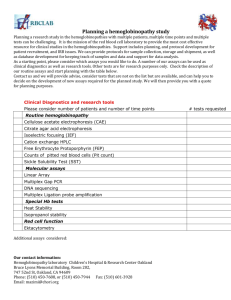
Supplementary File 5: Advantages and disadvantages of
advertisement

Hanekom: Immunol. outcomes of novel TB vaccine trials: WHO panel recommendations. 07-PLME-BP0785. Supplementary File S5. Advantages and disadvantages of different assay approaches for delineating T cell immunity. Page 1 of 6 Supplementary File S5: Advantages and disadvantages of different assay approaches for delineating T cell immunity. For specific details and references, please refer to Supplementary File S1. T cell assay Advantages Disadvantages approach Whole blood Smaller blood volumes are Incubation with antigens is required, compared with on a per-volume basis; T PBMC-based assays. cell depletion may Assessment of a whole therefore result in loss of immunological sensitivity and may require compartment is adjustment of blood accomplished. The volumes. measured T cell response Whole blood may be used will reflect cellular and undiluted when incubation soluble components that is ≤18 hours, but longer affect antigen presentation term incubation requires and T cell activation. dilution with RPMI, and The stimulation phase of therefore more resources. this approach requires The measured response relatively few resources; reflects not only T cells, assessment may often be but other peripheral blood completed in 2 phases: components also. For first immediate incubation example, when cytokines with antigen, followed by levels are determined in cryopreservation of plasma obtained from plasma, fixed white cells or whole blood, Hanekom: Immunol. outcomes of novel TB vaccine trials: WHO panel recommendations. 07-PLME-BP0785. Supplementary File S5. Advantages and disadvantages of different assay approaches for delineating T cell immunity. Page 2 of 6 RNA for later analysis, measurements may reflect e.g., soluble, cell- contributions of associated or mRNA neutrophils and expression analysis of monocytes. cytokine in the respective samples. The need for immediate processing of collected blood limits versatility in what may be measured. Freshly isolated PBMC PBMC-based assays are PBMC isolation requires incubated on a per-cell more resources, compared basis, resulting in with whole blood increased sensitivity in the stimulation. face of T cell depletion, PBMC isolation is such as in HIV infection, associated with compared with whole blood considerable cellular loss, assays. requiring larger blood PBMC isolation may allow volumes. for a description of T cell Incubation of PBMC often responses without the involves introduction of influence of other whole foreign material, such as blood components such as foetal calf or unrelated neutrophils and plasma. human serum; however, serum-free media may also be used. In short term assays, the cellular activation as a Hanekom: Immunol. outcomes of novel TB vaccine trials: WHO panel recommendations. 07-PLME-BP0785. Supplementary File S5. Advantages and disadvantages of different assay approaches for delineating T cell immunity. Page 3 of 6 result of PBMC isolation may result in higher backgrounds, compared with similar incubation of whole blood. Cryopreserved PBMC PBMC are available for The measured response in later assaying in batches, assays using which is of particular cryopreserved PBMC is importance for reducing invariably lower than when variability in longitudinal fresh PBMC or whole blood vaccination studies. is used. PBMC are available at a Optimal biobanking later date for application of procedures are critical for scientific advances not long-term storage of known when blood was PBMC, and require collected. This is very significant resources, such important in tuberculosis as LN2. vaccination studies, as the The specific protocol used immune correlates of for PBMC cryopreservation protection against may significantly impact tuberculosis are not yet on yield of viable, known. functional cells after thawing. Assays may produce more optimal results when Hanekom: Immunol. outcomes of novel TB vaccine trials: WHO panel recommendations. 07-PLME-BP0785. Supplementary File S5. Advantages and disadvantages of different assay approaches for delineating T cell immunity. Page 4 of 6 thawed PBMC are “rested” in medium for at least 6 hours, prior to incubation with antigens. Short term These assays are often Whole blood should ideally (<24hr, e.g., called “directly ex vivo” be incubated and PBMC ELISPOT assays, assays, meaning that isolated and incubated as intracellular results should reflect the in soon as possible after cytokine assays) vivo state of circulating T collection, to avoid loss of cells. Effector function of sensitivity. This appears to specific cells is assessed be of particular concern without a prolonged period when protein or whole of cell activation, not bacterial or viral antigens allowing time for cellular need to be processed, and division, and considered to may be less important for result in more quantitative peptide antigens. outcomes. For example, the frequency of antigenspecific T cells may be defined by cell-associated cytokine production or activation marker expression, as these T cells would not have divided during the short period of incubation. Hanekom: Immunol. outcomes of novel TB vaccine trials: WHO panel recommendations. 07-PLME-BP0785. Supplementary File S5. Advantages and disadvantages of different assay approaches for delineating T cell immunity. Page 5 of 6 Whole blood may be used undiluted. Intermediate and Expansion of cells may longer term assays increase sensitivity, i.e., (1-7 days, e.g., 48 ability to measure a hour ELIPOT, response not measurable lymphoproliferation with other systems. assays) Increased risk of contamination. Requires more resources, including CO2 incubators. Results are necessarily Both the presence of semi-quantitative, specific T cells and their reflecting phenotype and capacity to proliferate and function of expanded cells produce cytokines or other – these phenotypes may functional markers may be not have been the same at assessed. The expansion the onset of the assay. over the culture period may result in increased sensitivity, as “resting” T cells, or T cells that require more than just a short period of antigenpresentation to become activated, may expand. It has therefore been proposed that central memory cells may be more optimally measured with longer term assays, Hanekom: Immunol. outcomes of novel TB vaccine trials: WHO panel recommendations. 07-PLME-BP0785. Supplementary File S5. Advantages and disadvantages of different assay approaches for delineating T cell immunity. Page 6 of 6 compared with shorter term assays; however, the latter hypothesis remains unproven. Measures of the central memory response are currently thought to be critical for determining long-term vaccine efficacy. It appears that the time to incubation or PBMC isolation, after blood collection, is not as important for longer term T cell assays. The assays are also less affected by the manipulation at PBMC isolation and cryopreservation.