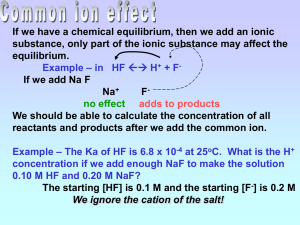
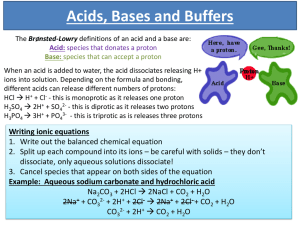
Buffer Calculations
advertisement
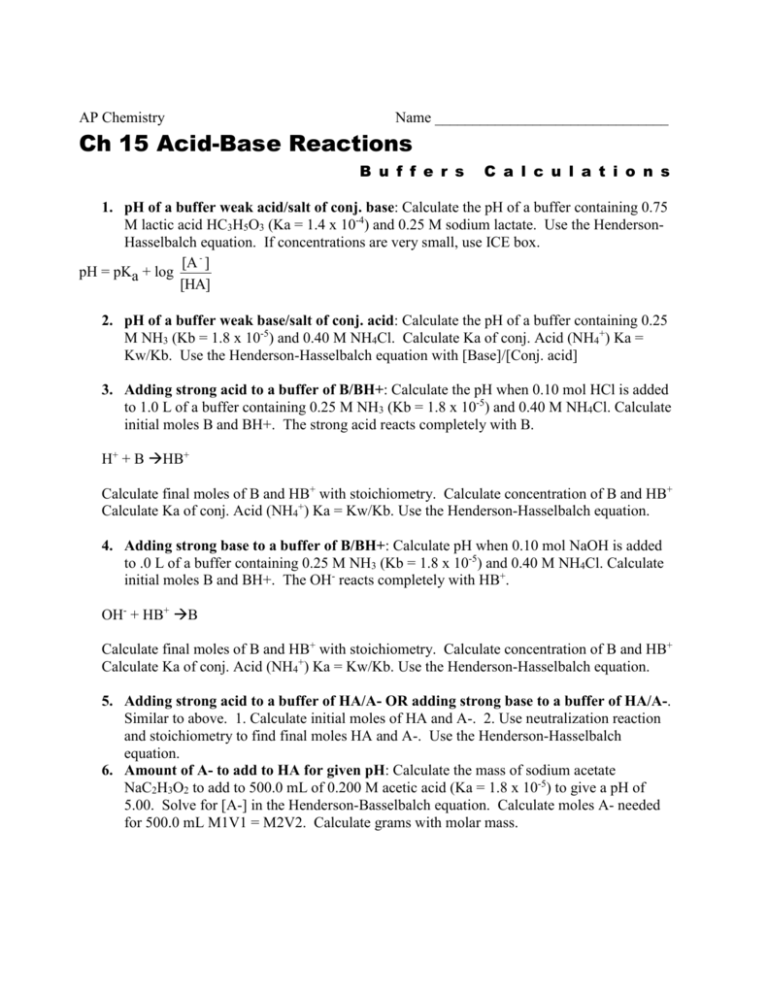
AP Chemistry Name _______________________________ Ch 15 Acid-Base Reactions B u f f e r s C a l c u l a t i o n s 1. pH of a buffer weak acid/salt of conj. base: Calculate the pH of a buffer containing 0.75 M lactic acid HC3H5O3 (Ka = 1.4 x 10-4) and 0.25 M sodium lactate. Use the HendersonHasselbalch equation. If concentrations are very small, use ICE box. [A - ] pH = pKa + log [HA] 2. pH of a buffer weak base/salt of conj. acid: Calculate the pH of a buffer containing 0.25 M NH3 (Kb = 1.8 x 10-5) and 0.40 M NH4Cl. Calculate Ka of conj. Acid (NH4+) Ka = Kw/Kb. Use the Henderson-Hasselbalch equation with [Base]/[Conj. acid] 3. Adding strong acid to a buffer of B/BH+: Calculate the pH when 0.10 mol HCl is added to 1.0 L of a buffer containing 0.25 M NH3 (Kb = 1.8 x 10-5) and 0.40 M NH4Cl. Calculate initial moles B and BH+. The strong acid reacts completely with B. H+ + B HB+ Calculate final moles of B and HB+ with stoichiometry. Calculate concentration of B and HB+ Calculate Ka of conj. Acid (NH4+) Ka = Kw/Kb. Use the Henderson-Hasselbalch equation. 4. Adding strong base to a buffer of B/BH+: Calculate pH when 0.10 mol NaOH is added to .0 L of a buffer containing 0.25 M NH3 (Kb = 1.8 x 10-5) and 0.40 M NH4Cl. Calculate initial moles B and BH+. The OH- reacts completely with HB+. OH- + HB+ B Calculate final moles of B and HB+ with stoichiometry. Calculate concentration of B and HB+ Calculate Ka of conj. Acid (NH4+) Ka = Kw/Kb. Use the Henderson-Hasselbalch equation. 5. Adding strong acid to a buffer of HA/A- OR adding strong base to a buffer of HA/A-. Similar to above. 1. Calculate initial moles of HA and A-. 2. Use neutralization reaction and stoichiometry to find final moles HA and A-. Use the Henderson-Hasselbalch equation. 6. Amount of A- to add to HA for given pH: Calculate the mass of sodium acetate NaC2H3O2 to add to 500.0 mL of 0.200 M acetic acid (Ka = 1.8 x 10-5) to give a pH of 5.00. Solve for [A-] in the Henderson-Basselbalch equation. Calculate moles A- needed for 500.0 mL M1V1 = M2V2. Calculate grams with molar mass.