Is it possible to turn coal into diamonds
advertisement
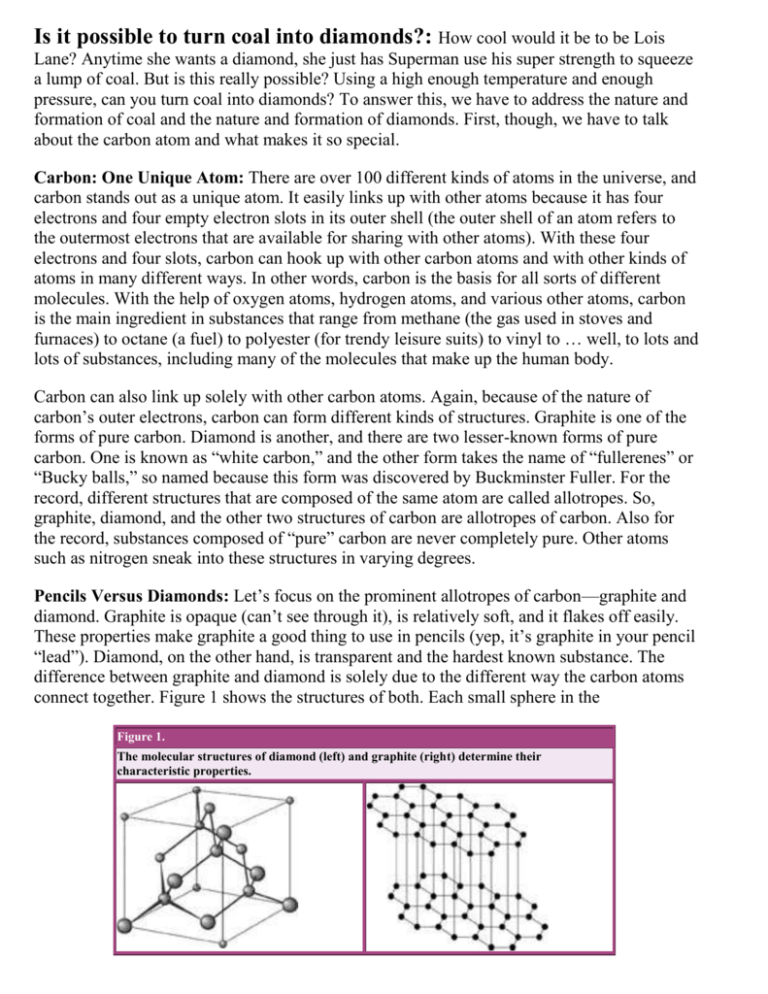
Is it possible to turn coal into diamonds?: How cool would it be to be Lois Lane? Anytime she wants a diamond, she just has Superman use his super strength to squeeze a lump of coal. But is this really possible? Using a high enough temperature and enough pressure, can you turn coal into diamonds? To answer this, we have to address the nature and formation of coal and the nature and formation of diamonds. First, though, we have to talk about the carbon atom and what makes it so special. Carbon: One Unique Atom: There are over 100 different kinds of atoms in the universe, and carbon stands out as a unique atom. It easily links up with other atoms because it has four electrons and four empty electron slots in its outer shell (the outer shell of an atom refers to the outermost electrons that are available for sharing with other atoms). With these four electrons and four slots, carbon can hook up with other carbon atoms and with other kinds of atoms in many different ways. In other words, carbon is the basis for all sorts of different molecules. With the help of oxygen atoms, hydrogen atoms, and various other atoms, carbon is the main ingredient in substances that range from methane (the gas used in stoves and furnaces) to octane (a fuel) to polyester (for trendy leisure suits) to vinyl to … well, to lots and lots of substances, including many of the molecules that make up the human body. Carbon can also link up solely with other carbon atoms. Again, because of the nature of carbon’s outer electrons, carbon can form different kinds of structures. Graphite is one of the forms of pure carbon. Diamond is another, and there are two lesser-known forms of pure carbon. One is known as “white carbon,” and the other form takes the name of “fullerenes” or “Bucky balls,” so named because this form was discovered by Buckminster Fuller. For the record, different structures that are composed of the same atom are called allotropes. So, graphite, diamond, and the other two structures of carbon are allotropes of carbon. Also for the record, substances composed of “pure” carbon are never completely pure. Other atoms such as nitrogen sneak into these structures in varying degrees. Pencils Versus Diamonds: Let’s focus on the prominent allotropes of carbon—graphite and diamond. Graphite is opaque (can’t see through it), is relatively soft, and it flakes off easily. These properties make graphite a good thing to use in pencils (yep, it’s graphite in your pencil “lead”). Diamond, on the other hand, is transparent and the hardest known substance. The difference between graphite and diamond is solely due to the different way the carbon atoms connect together. Figure 1 shows the structures of both. Each small sphere in the Figure 1. The molecular structures of diamond (left) and graphite (right) determine their characteristic properties. drawings represents a carbon atom. In graphite, the carbon atoms group together in layers, leading to its “flaky” property. In diamond, the carbon atoms connect in a shape known as a “tetrahedron” that results in an overall cubic shape. The structure of diamond is especially sturdy. This leads to graphite and carbon having drastically different hardness, and it leads to light interacting with them in drastically different ways. Graphite rings never caught on because they just don’t have that diamond sparkle. Natural diamonds form deep underground, anywhere from 120 km to 200 km beneath the surface of the Earth. There the temperature and pressure are high enough to assemble carbon atoms into the particular structure of diamonds. Volcanic activity then brings diamonds near enough to the surface of the Earth that we can mine them. Most of the diamonds we mine are not pure enough for use in jewelry, so they’re used in industry on diamond-tipped drills or saws (remember, diamonds are the hardest known substance). Of course, even gem-grade diamonds have impurities, and these impurities affect how light interacts with them. Completely pure diamond is clear, and diamonds with various impurities can be yellow, brown, or blue. The blue diamonds have many impurities, but they’re also very expensive. Why? Because they’re rare. Okay, let’s talk about creating diamonds artificially. Can you do it? Sure. At pressures and temperatures obtainable in the lab, we can take graphite and turn it into diamond. Makes sense, because graphite and diamond are both made of carbon atoms. Presently, artificial diamonds don’t quite have the same qualities as natural diamonds, so don’t start investing in pencil leads just yet. The process is improving, though, so it’s possible that one day a jeweler won’t be able to tell the difference between artificial and natural diamonds. So, What About Coal?: I haven’t said anything about coal yet, have I? That’s because coal is not an allotrope of carbon. Coal forms over millions of years from the decayed remains of living things. As such, coal contains a lot of carbon, but it is by no means close to pure carbon. It has many atoms of oxygen, nitrogen, and other atoms. Coal contains anywhere from 50% to 90% carbon. So, what happens if you use high temperatures and pressures to try and turn coal into diamond? You get lousy diamonds, with lots of impurities. Of course, coal is good for lots of other things, namely as a fuel for generating electricity and as an ingredient in the recipe for manufacturing many metals. Where does this leave Lois Lane? Unfortunately, she turns out to be a cheap date because she accepts low-grade diamonds produced from coal that the guy in the cape gives her. As soon as he switches to squeezing pencils, though, Lois will be in business.




