instructions to authors for the preparation - The Gibson Group
advertisement
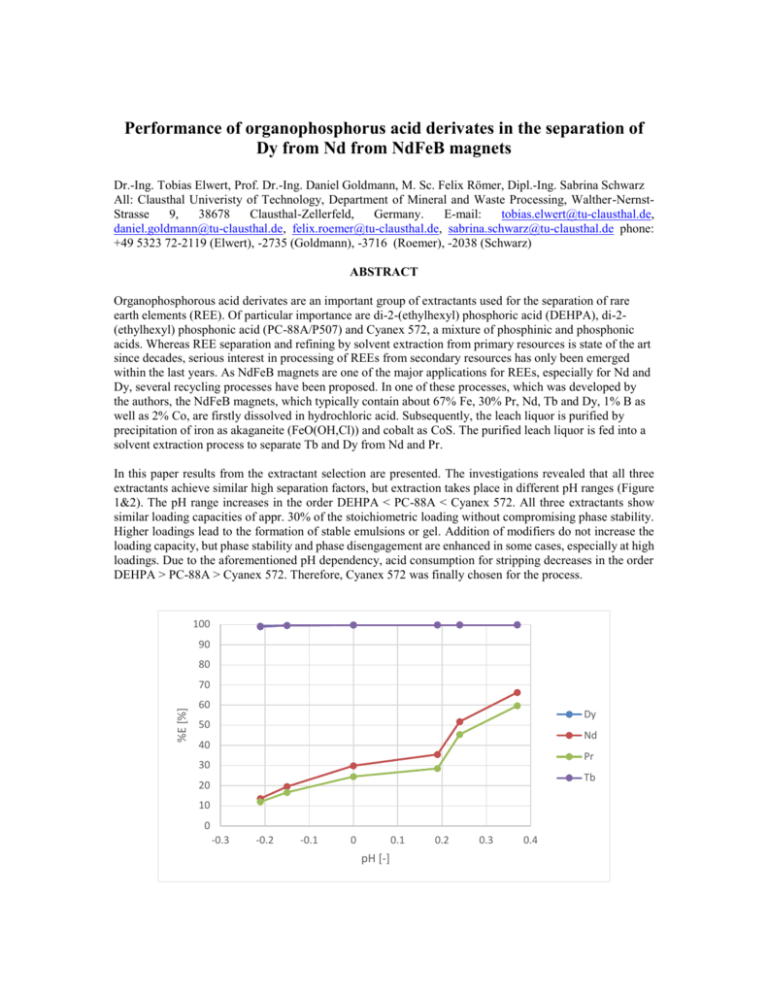
Performance of organophosphorus acid derivates in the separation of Dy from Nd from NdFeB magnets Dr.-Ing. Tobias Elwert, Prof. Dr.-Ing. Daniel Goldmann, M. Sc. Felix Römer, Dipl.-Ing. Sabrina Schwarz All: Clausthal Univeristy of Technology, Department of Mineral and Waste Processing, Walther-NernstStrasse 9, 38678 Clausthal-Zellerfeld, Germany. E-mail: tobias.elwert@tu-clausthal.de, daniel.goldmann@tu-clausthal.de, felix.roemer@tu-clausthal.de, sabrina.schwarz@tu-clausthal.de phone: +49 5323 72-2119 (Elwert), -2735 (Goldmann), -3716 (Roemer), -2038 (Schwarz) ABSTRACT Organophosphorous acid derivates are an important group of extractants used for the separation of rare earth elements (REE). Of particular importance are di-2-(ethylhexyl) phosphoric acid (DEHPA), di-2(ethylhexyl) phosphonic acid (PC-88A/P507) and Cyanex 572, a mixture of phosphinic and phosphonic acids. Whereas REE separation and refining by solvent extraction from primary resources is state of the art since decades, serious interest in processing of REEs from secondary resources has only been emerged within the last years. As NdFeB magnets are one of the major applications for REEs, especially for Nd and Dy, several recycling processes have been proposed. In one of these processes, which was developed by the authors, the NdFeB magnets, which typically contain about 67% Fe, 30% Pr, Nd, Tb and Dy, 1% B as well as 2% Co, are firstly dissolved in hydrochloric acid. Subsequently, the leach liquor is purified by precipitation of iron as akaganeite (FeO(OH,Cl)) and cobalt as CoS. The purified leach liquor is fed into a solvent extraction process to separate Tb and Dy from Nd and Pr. In this paper results from the extractant selection are presented. The investigations revealed that all three extractants achieve similar high separation factors, but extraction takes place in different pH ranges (Figure 1&2). The pH range increases in the order DEHPA < PC-88A < Cyanex 572. All three extractants show similar loading capacities of appr. 30% of the stoichiometric loading without compromising phase stability. Higher loadings lead to the formation of stable emulsions or gel. Addition of modifiers do not increase the loading capacity, but phase stability and phase disengagement are enhanced in some cases, especially at high loadings. Due to the aforementioned pH dependency, acid consumption for stripping decreases in the order DEHPA > PC-88A > Cyanex 572. Therefore, Cyanex 572 was finally chosen for the process. 100 90 80 %E [%] 70 60 Dy 50 Nd 40 Pr 30 Tb 20 10 0 -0.3 -0.2 -0.1 0 0.1 pH [-] 0.2 0.3 0.4 Figure 1 – pH dependence of the extraction of Dy, Nd, Pr and Tb with 1M DEHPA in Exxsol D100 at an A:O ratio of 1:1 and a contact time of 300 s from a hydrochloric acid solution 100 90 80 %E [%] 70 60 Dy 50 Nd 40 Pr 30 Tb 20 10 0 0 0.5 1 1.5 2 pH [-] Figure 2 – pH dependence of the extraction of Dy, Nd, Pr and Tb with 1M Cyanex 572 in Exxsol D100 at an A:O ratio of 1:1 and a contact time of 300 s from a hydrochloric acid solution KEYWORDS Rare earth, recycling, solvent extraction, NdFeB magnets, DEHPA, PC-88A, Cyanex 572