Chemotherapy
advertisement

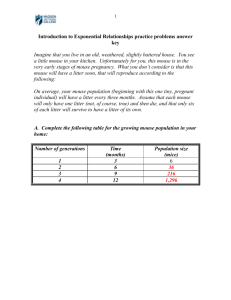
SOP Chemotherapy Starr Lab PI: Timothy K. Starr Lab Location: 12-175 Moos Towers Original issue date: 4/16/14 Revision dates: Prepared by: Michelle Glasgow and Timothy K. Starr Approved by: Timothy K. Starr Table of Contents Table of Contents ................................................................................................................................................ 1 Overview ................................................................................................................................................................ 1 Background........................................................................................................................................................... 1 Antimetabolites ............................................................................................................................................................... 2 Plant derivatives ............................................................................................................................................................. 2 Alkylating agents............................................................................................................................................................. 2 Targeted chemotherapeutics ..................................................................................................................................... 2 Procedures ............................................................................................................................................................ 2 General safety procedures........................................................................................................................................... 2 Preparation of chemotherapies................................................................................................................................. 3 Dosing of chemotherapies ........................................................................................................................................... 3 Intra-peritoneal (IP) injection of chemotherapies ............................................................................................. 3 Intra-venous (IV) injection of chemotherapies in tail vein ............................................................................. 4 Oral gavage administration of chemotherapies .................................................................................................. 4 Hazard Identification and Risk of Exposure to the Hazards: .............................................................. 6 Exposure Controls Specific to Above Risk of Exposure: .................................................................................... 6 Waste Generated and Disposal Methods .................................................................................................... 6 Spill and Accident Response Procedures ................................................................................................... 6 Further sources of information ..................................................................................................................... 7 References ............................................................................................................................................................. 8 Overview This protocol covers procedures related to delivery of chemotherapeutic agents to mice. Background Chemotherapy agents fall into different classifications, including: antimetabolites, plant derivatives, topoisomerase inhibitors, alkylating agents, and antibiotics, among others. The chemotherapeutic agents covered by this protocol are discussed below based on their main mode of action. Antimetabolites 5-fluorouracil (5-FU) is classified as an antimetabolite. Antimetabolites act by inhibiting essential metabolic processes that are required for synthesis of purines, pyrimidines, and nucleic acids. Antimetabolites are typically S phase-specific. Specifically, 5-FU acts through inhibition of thymidylate synthase, which is required for synthesis of pyrimidine thymidine, a nucleoside required for DNA replication. Following administration of 5-FU, there are insufficient levels of thymidine monophosphate (dTMP) and rapidly dividing cancerous cells undergo cell death. Plant derivatives Taxol is a chemotherapy agent derived from a plant. Taxol was originally isolated from the bark of the Pacific yew tree, however, it is now produced using plant cell fermentation technology. Its main mechanism is to stabilize microtubules such that they cannot disassemble during cell division. As a result, chromosomes are unable to achieve a metaphase spindle configuration and mitosis cannot progress. Alkylating agents Carboplatin is one of the most commonly used alkylating agents. It prevents cell division by crosslinking strands of DNA. Cell death results because of continued synthesis of other cell constituents, such as RNA and protein, which leads to unbalanced cell growth and ultimately cell death. Targeted chemotherapeutics NVP-BKM120 is a compound that inhibits phosphoinosotide 3-kinase (PI3K), a lipid kinase that consists of enzymatic subunits that are subdivided, on the basis of sequence homology and substrate specificity, into class I, II, and III and the p85/p55 regulatory subunits. Activating and transforming mutations in the PIK3CA gene of the p110α subunit (one of the subgroups of class I) of PI3K are commonly found in cancer. NVP-BKM120 can inhibit all four Class I PI3K isoforms, as well as the more common somatic PI3K mutations (H1047R, E542K and E545K). Administration of NVP-BKM120 leads to inhibition of various Akt downstream signaling pathway components. Studies have shown that this compound demonstrates significant, concentration dependent, cell growth inhibition and induction of apoptosis in a variety of tumor cancer cells, particularly for those harboring p110 mutants and/or over-expressing erbB2. Antibodies targeting vascular endothelial growth factor (VEGF) are used to treat cancer. VEGFVEGFR signaling has been identified as a major regulator of endothelial cell growth and survival and has been implicated in pathological angiogenesis. Monoclonal antibodies (mAbs) that bind to VEGF and block this signaling have been developed for the purpose of inhibition of tumor growth. Procedures General safety procedures Working stocks of chemotherapeutics will be prepared inside a Biological Safety Cabinet. Always wear PPE including lab coat, gloves, facemask and eye goggles when handling and preparing stock solutions of chemotherapeutics. RAR Area Supervisor must be informed of your intent to use chemotherapies. PI must train RAR staff on hazards and proper handling procedures. Cages containing animals injected with chemotherapies must be clearly labeled as such. Preparation of chemotherapies All chemotherapeutic agents will be prepared following manufacturer's guidelines. Stock solutions will be sterilized when appropriate. In general, drugs will be delivered in the following vehicles: 5-FU in 0.9% NaCl (saline solution) Carboplatin in 0.9% NaCl (saline solution) Taxol in 50% cremophor/50% ethanol NVP-BKM120 in N-Methyl-2-pyrrolidone (NMP) and Polyethylene Glycol 300 (10%/90% v/v/) Antibodies to VEGF in 0.9% NaCl (saline solution) Dosing of chemotherapies Dosing of chemotherapies is generally based on existing literature reporting use of the chemotherapies in mice. In combination trials, experimental protocols will specify the dosing range and the combination dosing range, generally based on in vitro assays previously used to determine ED50s of the drugs. 5-FU: Multiple dosing strategies have been used in mice including: 1) 50 mg/kg for 4 consecutive days (Farrell, Bready et al. 1998); 2) 40 mg/kg IP daily bolus for 2-week cycles of 5 days on followed by 9 days off, continuing until euthanization or moribundity (Durkee, Shinki et al. 2009); 3) Single IP bolus of 400 mg/kg (Boushey, Yusta et al. 2001); and 4) 12.5 mg/kg in HBSS IV daily for 2 days in a xenograft model (Bunz, Hwang et al. 1999). The maximum tolerable dose in nude mice is 100 mg/kg/week x 4 weeks (Azrak, Cao et al. 2004). In our studies we will start with 40 mg/kg administered IP daily for 2-week cycles of 5 days on followed by 9 days off, based on the work by (Durkee, Shinki et al. 2009). Taxol: Multiple dosing strategies have been used in mice including: 1) 12 mg/kg IV tail vein once every 2 days for a total of three doses (Roby, Niu et al. 2008); and 2) 10 mg/kg IP every 2 days continuously until moribund or endpoint (Campbell, Greenaway et al. 2010). The maximum tolerable dose in mice is 80 mg/kg in C3Hf/Kam mice (Li, Price et al. 1999) and 60 mg/kg administered IV as 20 mg/kg per day for three days in nude mice (Huang, Lopez-Barcons et al. 2006). In our studies we will start with 10 mg/kg administered IP every 2 days based on the work by (Campbell, Greenaway et al. 2010). Carboplatin: Based on the work of (Farrell, Bready et al. 1998) we will start with 125 mg/kg administered IV for one day for non-lethal treatment. The maximum tolerable dose in nude mice is between 120 and 160 mg/kg when administered IV once per week for two weeks (Boven, van der Vijgh et al. 1985) The LD(10) for mice is 165 mg/kg after a single IV bolus injection (van Hennik, van der Vijgh et al. 1987). NVP-BKM120: Based on the work of (Maira, Pecchi et al. 2012) we will use a single dose of 30-60 mg/kg administered by oral gavage. Anti-VEGF antibodies: Based on the work of (Jiang, Engelbach et al. 2014) we will use the anti-VEGF monoclonal antibody, B20-4.1.1 (Genentch), at 10mg/kg administered IP twice per week. Intra-peritoneal (IP) injection of chemotherapies Prepare working stock of chemotherapeutic by diluting stock solution to the appropriate concentration using sterile vehicle solution. Load a 1 ml syringe (23 gauge needle) with appropriate volume of working stock. Keep in sterile field. Hold mouse firmly: Place mouse on a rough surface while holding the tail firmly (Note: Smooth surfaces will frighten the mouse because it cannot get a foothold. This may cause it to turn around and try to bite in its attempt to escape.) Grasp the nape gently and firmly with your free hand and lift the mouse. Place the mouse's tail between the last two fingers of the hand that is holding the nape. (See Figure below). Insert needle 10 mm ventral to hip joint. Insert needle through skin/peritoneum into stomach cavity. Avoid nipples and keep needle close to skin to avoid perforating viscera. Inject up to 100 µl of working stock solution. Dispose of needles immediately in sharps containers. Replace mouse in cage. Figure: Method of immobilizing mouse for IP injection Intra-venous (IV) injection of chemotherapies in tail vein Place mouse securely in harness. Dip tail in warm water (45ºC) for 30 seconds. Wipe dry with sterile gauze Insert needle into lateral tail vein and gently push plunger (see Figure below). Remove needle, remove mouse from harness, and return mouse to cage. A B Figure Proper technique for IV injections. A) Warm tail by dipping in 45ºC water for 30 seconds, then wipe with sterile gauze. B) Insert needle in lateral tail vein. Oral gavage administration of chemotherapies The following procedure is based on the protocol described in (Bertola, Mathews et al. 2013). See figure below as a guide. Select the appropriate gavage needle gauge size and length based on the size and weight of animals. For example, 15–20 g mice: 22-gauge (1–1.5 inches); 20–25 g mice: 20-gauge (1–1.5 inches); 25–30 g mice: 18-gauge (1.5–2 inches). Attach gavage needle with ball tip to 1 ml syringe and draw up the appropriate volume of chemotherapy. Volumes administered should not exceed 2 mls per 100 g body weight. Gently grasp mouse as described in the IP injections above. Place needle in mouth between incisors and molars and pass needle along roof of mouth while head is held in moderate extension. Gently extend head and neck backwards using the needle as a lever and position the neck and esophagus in a straight line. Move the gavage needle smoothly along the upper palate into the esophagus. The mouse generally will initiate a swallowing reflex as the needle nears the pharynx, which facilitates entry into the esophagus. Gently push the syringe plunger and expelling the liquid into the mouse's stomach Slowly remove the needle by following the same motion used to insert the needle. Figure: Oral gavage technique. a) measure length of the gavage needle against the animal's body. b) gently insert needle through mouth and esophagus into stomach. c) gently expel chemotherapy using the syringe plunger, then remove needle Return the mouse to its cage and monitor the mouse for at least 10 minutes for any sign of labored breathing or respiratory distress. If mouse displays any signs of respiratory distress, such as frothing at the mouth or nose, or difficulty in breathing, the mouse may have aspirated or the trachea or esophagus may have been perforated. If these signs persist for more than one to two minutes after removing the needle, the mouse will be euthanized. Tips for successful oral gavage: Place needle behind the base of tongue near the center of the back of the mouth. Avoid the side of the mouth where mouse can bite needle. Wait until the animal begins to swallow due to a swallowing reflex, which allows the needle to "fall" down esophagus with little or no pressure. Make sure needle is in stomach, not trachea, by checking the depth of insertion. You can also pull the plunger back a small distance and if you see air bubbles, that indicates the needle is in the trachea, not the stomach. Never use excessive pressure, which could perforate the mouth, or esophagus. Hazard Identification and Risk of Exposure to the Hazards: Sharps, especially needles used for injection. Never recap needles, but place directly in Sharps container. Exposure to chemotherapeutics via accidental spills, splashes, inhalation or needle sticks could cause adverse health reactions. Exposure Controls Specific to Above Risk of Exposure: PPE - Lab coats, safety goggles, mask, gloves, sleeves and sharps containers. Use Biologic Safety Cabinet during preparation and handling of chemotherapeutic agents. All sharps and glass waste will be disposed of in an approved hard plastic sharps container (U Stores # CX40245, MS07407 or similar), No Cardboard sharps pouches. If exposure occurs: Consult a physician. Show the MSDS to the doctor in attendance. Move out of dangerous area. If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact wash off with soap and plenty of water. Consult a physician. In case of eye contact flush eyes with water as a precaution. If swallowed never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. In case of accidental injection seek medical attention immediately. Waste Generated and Disposal Methods Chemotherapies are considered hazardous chemicals. Any unused chemotherapies that need to be discarded should be placed in a properly labeled hazardous waste container and disposed of by the University of Minnesota Chemical Waste Facility (part of the Division of Environmental Health & Safety). Sharps containers will be sealed when ¾ full and placed in designated waste area. In addition to unused chemical waste, the following waste disposal measures should be followed: Sharps contaminated with trace carcinogens or hazardous drugs must be disposed of in a separate sharps container that is labeled “Hazardous Sharps,” sealed, and placed in a yellow bag for incineration Residual and trace chemotherapeutic wastes, such as completely empty delivery devices, gowns, gloves, tubing, etc., should be disposed of in yellow bags for incineration. Bedding should be put in yellow bags for collection and managed as hazardous chemical waste (only while being treated with chemotherapies) Spill Response Procedures Clean up waste regularly and abnormal spills immediately. Avoid breathing dust and avoid contact with skin and eyes. Wear protective clothing, gloves, safety glasses and dust respirator. Use dry clean up procedures and avoid generating dust. Vacuum up or sweep up. NOTE: Vacuum cleaner must be fitted with an exhaust micro filter (HEPA type) (consider explosion-proof machines designed to be grounded during storage and use). Dampen with water to prevent dusting before sweeping. Place in suitable containers for disposal. If in liquid form, wipe up with paper towel. When decontaminating is finished carefully place any and all materials in appropriate biohazard bin/bag or autoclave bag. Only attempt to clean up very small powder and liquid spills (e.g., very small amounts spilled around a balance). In this situation, wear appropriate PPE as described, place wet paper towels over powder or use absorbant pads for liquid, and place all materials in leak proof container. All spill materials should be disposed of as hazardous waste through the U of M Chemical Waste Program. Larger spills should be immediately reported to DEHS for cleanup (612-626-6002). Accident Response Procedures If Incident Results in a Hazard Exposure ( i.e. face or eye splash, cut or puncture with sharps, contact with non-intact skin): Encourage needle sticks and cuts to bleed, gently wash with soap and water for 5 minutes; flush splashes to the nose, mouth, or skin with water; and flush eyes at the nearest eyewash station with clean water for 15 minutes. Call 911 or seek medical attention. o For urgent care employees may go to HealthPartners Occupational and Environmental Medicine (M/F day time or Urgent Care after hours), or UMMC-Fairview Hospital (24 hrs). You may seek medical attention at the closest available medical facility or your own healthcare provider. o Follow-up must be done by HealthPartners Occupational and Environmental Medicine. Report the incident to your supervisor as soon as possible, fill out the appropriate documentation. o Employee First Report of Injury o Supervisor Incident Investigation Report Send Incident Report Form to the IBC if exposure has occurred during work on an IBC protocol. Report all biohazard exposures to the Office of Occupational Health and Safety (612-626-5008) or uohs@umn.edu. Note: It is important to fill out all of the appropriate documents to be eligible to collect workers compensation should any complications from the hazardous exposure arise in the future. Further sources of information For further information view the UMN DEHS website containing Bio Basic Fact Sheets at http://www.dehs.umn.edu/bio_basicfacts.htm. For general information on Biosafety, access the Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition from the CDC at BMBL http://www.cdc.gov/biosafety/publications/bmbl5/index.htm For Material Safety Data Sheets access the Public Health agency of Canada website MSDS http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/index-eng.php. References Azrak, R. G., S. Cao, H. K. Slocum, K. Toth, F. A. Durrani, M. B. Yin, L. Pendyala, W. Zhang, H. L. McLeod and Y. M. Rustum (2004). "Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts." Clin Cancer Res 10(3): 1121-1129. Bertola, A., S. Mathews, S. H. Ki, H. Wang and B. Gao (2013). "Mouse model of chronic and binge ethanol feeding (the NIAAA model)." Nat Protoc 8(3): 627-637. Boushey, R. P., B. Yusta and D. J. Drucker (2001). "Glucagon-like peptide (GLP)-2 reduces chemotherapy-associated mortality and enhances cell survival in cells expressing a transfected GLP-2 receptor." Cancer Res 61(2): 687-693. Boven, E., W. J. van der Vijgh, M. M. Nauta, H. M. Schluper and H. M. Pinedo (1985). "Comparative activity and distribution studies of five platinum analogues in nude mice bearing human ovarian carcinoma xenografts." Cancer Res 45(1): 86-90. Bunz, F., P. M. Hwang, C. Torrance, T. Waldman, Y. Zhang, L. Dillehay, J. Williams, C. Lengauer, K. W. Kinzler and B. Vogelstein (1999). "Disruption of p53 in human cancer cells alters the responses to therapeutic agents." The Journal of clinical investigation 104(3): 263-269. Durkee, B. Y., K. Shinki, M. A. Newton, C. E. Iverson, J. P. Weichert, W. F. Dove and R. B. Halberg (2009). "Longitudinal assessment of colonic tumor fate in mice by computed tomography and optical colonoscopy." Acad Radiol 16(12): 1475-1482. Farrell, C. L., J. V. Bready, K. L. Rex, J. N. Chen, C. R. DiPalma, K. L. Whitcomb, S. Yin, D. C. Hill, B. Wiemann, C. O. Starnes, A. M. Havill, Z. N. Lu, S. L. Aukerman, G. F. Pierce, A. Thomason, C. S. Potten, T. R. Ulich and D. L. Lacey (1998). "Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality." Cancer Res 58(5): 933-939. Jiang, X., J. Engelbach, L. Yuan, J. Cates, F. Gao, R. E. Drzymala, D. E. Hallahan, K. M. Rich, R. E. Schmidt, J. H. Ackerman and J. R. Garbow (2014). "Anti-VEGF antibodies mitigate the development of radiation necrosis in mouse brain." Clin Cancer Res. Maira, S. M., S. Pecchi, A. Huang, M. Burger, M. Knapp, D. Sterker, C. Schnell, D. Guthy, T. Nagel, M. Wiesmann, S. Brachmann, C. Fritsch, M. Dorsch, P. Chene, K. Shoemaker, A. De Pover, D. Menezes, G. Martiny-Baron, D. Fabbro, C. J. Wilson, R. Schlegel, F. Hofmann, C. Garcia-Echeverria, W. R. Sellers and C. F. Voliva (2012). "Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor." Mol Cancer Ther 11(2): 317-328. van Hennik, M. B., W. J. van der Vijgh, I. Klein, F. Elferink, J. B. Vermorken, B. Winograd and H. M. Pinedo (1987). "Comparative pharmacokinetics of cisplatin and three analogues in mice and humans." Cancer Res 47(23): 6297-6301.