Kassanova AZh en
advertisement

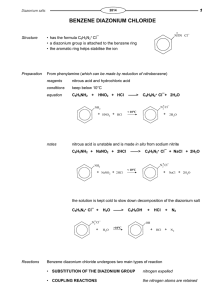
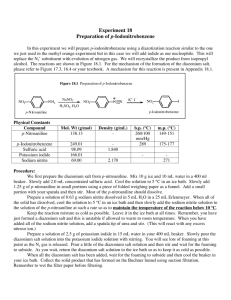
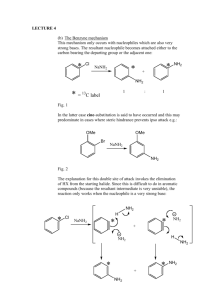
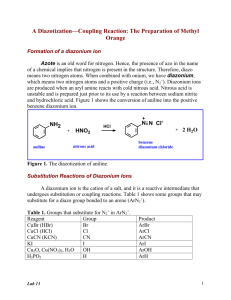
Diazotization of aminoheterocycles in trifluoromethanesulfonic acid presence Assiya Zh. Kassanova*, Ol'ga A. Firstova, Elena A. Krasnokutskaya Department of Biotechnology and Organic Chemistry, National Research Tomsk Polytechnic UniversityTomsk polytechnic university, Lenina Avenue, 30, 634050, Tomsk, Russia * E-mail: asiyakass@mail.ru Heterocyclic diazonium salts are valuable intermediates in organic synthesis. Despite the great practical importance of these compounds, there are still no reliable methods for their preparation [1]. Recently we reported a new method of aryldiazonium triflates (ArN2+ OTf-) (storage-stable, explosion proof diazonium salts) synthesis via diazotization of wide range of anilines by using butylnitrite (n-Bu-ONO) in the presence of trifluoromethanesulfonic acid (TfOH) in acetic acid [2]. It was shown that after aminopyridines (1 a – 2-aminopyridine, 1 b – 3-aminopyridine, 1 c – 4-aminopyridine) and 2-aminoquinoline (1d) treatment by n-Bu-ONO / TfOH system in the presence of AcOH they are diazotized, but instead of expected corresponding diazonium salts pyridin triflates are formed. Diazotization of π-excessive aminoheterocycles (1 e - quinolin-6-amine, 1 f - thiazol-2amine, 1 g - 1H-tetrazol-5-amine, 1 h - 1H-benzo[d]imidazol-2-amine, 1 i - 6- methylbenzo[d]thiazol-2-amine, 1 j - 1H-indazol-6-amine, 1 k - 1,1'-(propane-1,3-diyl)bis(3,5dimethyl-1H-pyrazol-4-amine)) in the conditions described above leads to formation of corresponding diazonium salts, which were identified by using iodo- and azido derivatives. References: 1 R. N. Butler. Diazotization of heterocyclic primary amines // Chem. Rev. -1975, V. 75, № 2, - p. 241-257 2 А. Zh. Kassanovа, М. Т. Estaeva, А. G. Fеfеlоvа. Synthesis and research aryldiazonium triflate // High technology in modern science and technology: collection of scientific papers III International scientific and technical conference of young scientists, postgraduates and students / Tomsk: Publisher.: TPU , 2014 . — 250 – 252.