Essential Questions
advertisement
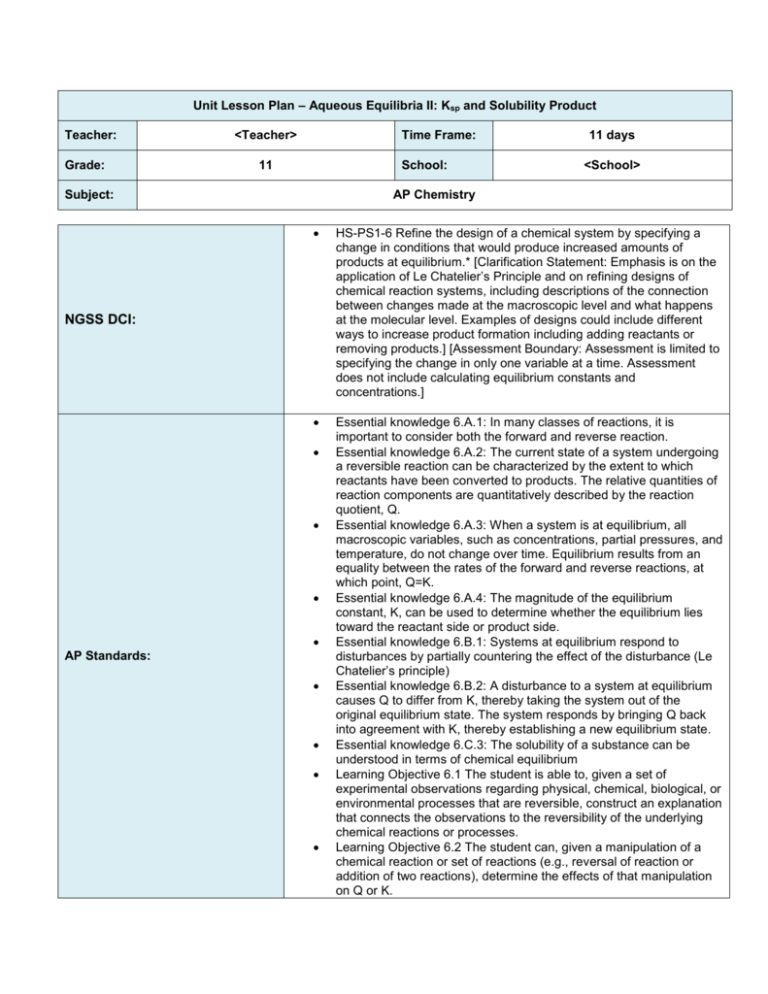
Unit Lesson Plan – Aqueous Equilibria II: Ksp and Solubility Product Teacher: Grade: <Teacher> Time Frame: 11 School: Subject: 11 days <School> AP Chemistry HS-PS1-6 Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium.* [Clarification Statement: Emphasis is on the application of Le Chatelier’s Principle and on refining designs of chemical reaction systems, including descriptions of the connection between changes made at the macroscopic level and what happens at the molecular level. Examples of designs could include different ways to increase product formation including adding reactants or removing products.] [Assessment Boundary: Assessment is limited to specifying the change in only one variable at a time. Assessment does not include calculating equilibrium constants and concentrations.] Essential knowledge 6.A.1: In many classes of reactions, it is important to consider both the forward and reverse reaction. Essential knowledge 6.A.2: The current state of a system undergoing a reversible reaction can be characterized by the extent to which reactants have been converted to products. The relative quantities of reaction components are quantitatively described by the reaction quotient, Q. Essential knowledge 6.A.3: When a system is at equilibrium, all macroscopic variables, such as concentrations, partial pressures, and temperature, do not change over time. Equilibrium results from an equality between the rates of the forward and reverse reactions, at which point, Q=K. Essential knowledge 6.A.4: The magnitude of the equilibrium constant, K, can be used to determine whether the equilibrium lies toward the reactant side or product side. Essential knowledge 6.B.1: Systems at equilibrium respond to disturbances by partially countering the effect of the disturbance (Le Chatelier’s principle) Essential knowledge 6.B.2: A disturbance to a system at equilibrium causes Q to differ from K, thereby taking the system out of the original equilibrium state. The system responds by bringing Q back into agreement with K, thereby establishing a new equilibrium state. Essential knowledge 6.C.3: The solubility of a substance can be understood in terms of chemical equilibrium Learning Objective 6.1 The student is able to, given a set of experimental observations regarding physical, chemical, biological, or environmental processes that are reversible, construct an explanation that connects the observations to the reversibility of the underlying chemical reactions or processes. Learning Objective 6.2 The student can, given a manipulation of a chemical reaction or set of reactions (e.g., reversal of reaction or addition of two reactions), determine the effects of that manipulation on Q or K. NGSS DCI: AP Standards: Note that this exact Smart Notebook presentation has not been used in the classroom, although all of the material has. The pacing below is approximate based on a 40-45 minute class period. Feel free to adjust as necessary and please provide your feedback! Learning Objective 6.3 The student can connect kinetics to equilibrium by using reasoning about equilibrium, such as Le Chatelier’s principle, to infer the relative rates of the forward and reverse reactions. Learning Objective 6.4 The student can, given a set of initial conditions (concentrations or partial pressures) and the equilibrium constant, K, use the tendency of Q to approach K to predict and justify the prediction as to whether the reaction will proceed toward products or reactants as equilibrium is approached. Learning Objective 6.5 The student can, given data (tabular, graphical, etc.) from which the state of a system at equilibrium can be obtained, calculate the equilibrium constant, K. Learning Objective 6.6 The student can, given a set of initial conditions (concentrations or partial pressures) and the equilibrium constant, K, use stoichiometric relationships and the law of mass action (Q equals K at equilibrium) to determine qualitatively and/or quantitatively the conditions at equilibrium for a system involving a single reversible reaction. Learning Objective 6.7 The student is able, for a reversible reaction that has a large or small K, to determine which chemical species will have very large versus very small concentrations at equilibrium. Learning Objective 6.8 The student is able to use Le Chatelier’s principle to predict the direction of the shift resulting from various possible stresses on a system at chemical equilibrium. Learning Objective 6.9 The student is able to use Le Chatelier’s principle to design a set of conditions that will optimize a desired outcome, such as product yield. Learning Objective 6.10 The student is able to connect Le Chatelier’s principle to the comparison of Q to K by explaining the effects of the stress on Q and K. Learning Objective 6.21 The student can predict the solubility of a salt, or rank the solubility of salts, given the Ksp relevant values Learning Objective 6.22 The student can interpret data regarding solubility of salts to determine, or rank, the Ksp relevant values. Learning Objective 6.23 The student can interpret data regarding the relative solubility of salts in terms of factors (common ions, pH) that influence the solubility. Essential Questions (What questions will the student be able to answer as a result of the instruction?) How can the solubility rules be used to give a qualitative prediction as to whether a precipitate will form? What is the difference between solubility and the solubility-product constant for a substance? How do we use solubility product, Ksp, to make quantitative predictions about solubility? What factors affect the solubility of a slightly soluble salt? How can Ksp be used to selectively precipitate ions? Knowledge & Skills (What skills are needed to achieve the desired results?) By the end of this unit, students will know: How to explain the meaning of saturated solution and the relation of solubility to Ksp. How to solve for the other two quantities given the Ksp, molar solubility or mass solubility for a substance. How to use Le Chatelier’s principle to reason qualitatively about the position of an equilibrium after a disturbance to the equilibrium has occurred. How the common ion effect and pH sensitivity effect solubility. Predict whether a precipitate will form when solutions are mixed by comparing Q and Ksp values. Calculate the ion concentrations required to begin precipitation and predict which ions will precipitate first. By the end of this unit, students will be able to: Rank the solubility of various salts using Ksp for salts with identical numbers of ions. Interconvert between Ksp, molar solubility and mass solubility for a substance. Determine the impact on the solubility of slightly soluble salt after the addition of a common ion. Determine the impact of changes in pH to a solution of a slightly soluble salt that has a basic or acidic ion. Predict whether or not a precipitate will form given the Ksp of a slightly soluble salt and the concentration of its constituent ions. Determine which ion will precipitate first given the Ksp of different slightly soluble salts and the concentration of constituent ions. Determine the concentration of the first ion to precipitate when the second ion begins to precipitate in a solution containing different ions. Assessment (What is acceptable evidence to show desired results (rubrics, exam, etc.)? Attach Copy During the Smart Notebook lesson designed to introduce concepts, students will be continually questioned on these concepts using a combination of class work/homework questions and the SMART Response system. Classwork and Homework questions will be discussed as a class and misconceptions will be addressed by the teacher prior to the formal evaluations listed below. Molar Solubility & Ksp Quiz Factors that Affect Solubility Quiz Selective Precipitation Quiz Ksp and Solubility Product Test Other assessments on the NJCTL website are optional and can be used as needed. (What is the sequence of activities, learning experiences, etc, that will lead to desired results (the plan)? Day 1 www.njctl.org Topic Classwork Homework** Introduction to Aqueous Equilibria: Ksp & Solubility Products 1-3 4-6 AP Chemistry Ksp and Solubility Product Note that this exact Smart Notebook presentation has not been used in the classroom, although all of the material has. The pacing below is approximate based on a 40-45 minute class period. Feel free to adjust as necessary and please provide your feedback! 2 Calculating Solubility from Ksp 7-10 11-14 3 Calculating Solubility from Ksp 15-18 19-22 4 Factors Affecting Solubility: Common Ion Effect 23-26 27-30 5 Factors Affecting Solubility: Changes in pH 31-34 35-38 7 Precipitation Reactions 39-41 42-44 8 Selective Precipitation 45-48 49-52 9 Conceptual Questions Review 1-10 11-20 10 Free Response Review 1-4 5-8 11 Ksp and Solubility Product Test Test N/A * It may not be possible to complete labs in the order stated due to lab schedules. Other labs on the NJCTL website are option and can be used as needed. **HW Problems are currently not scaffolded from least to most difficult, but are instead listed in order of topic. Teacher should pay special attention at the end of each class period when assigning HW so that only problems related to the topic that was taught are being assigned.