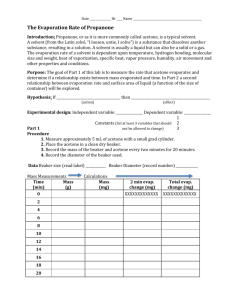
Lab 16 - Kinetics
advertisement
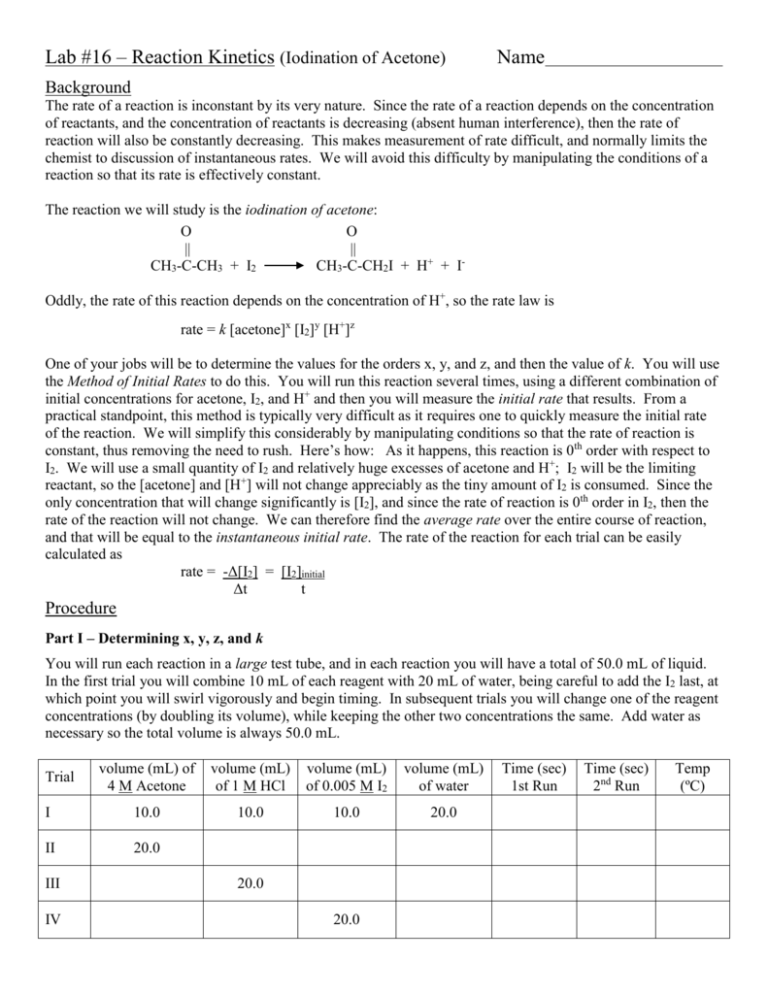
Lab #16 – Reaction Kinetics (Iodination of Acetone) Name Background The rate of a reaction is inconstant by its very nature. Since the rate of a reaction depends on the concentration of reactants, and the concentration of reactants is decreasing (absent human interference), then the rate of reaction will also be constantly decreasing. This makes measurement of rate difficult, and normally limits the chemist to discussion of instantaneous rates. We will avoid this difficulty by manipulating the conditions of a reaction so that its rate is effectively constant. The reaction we will study is the iodination of acetone: O O || || CH3-C-CH3 + I2 CH3-C-CH2I + H+ + IOddly, the rate of this reaction depends on the concentration of H+, so the rate law is rate = k [acetone]x [I2]y [H+]z One of your jobs will be to determine the values for the orders x, y, and z, and then the value of k. You will use the Method of Initial Rates to do this. You will run this reaction several times, using a different combination of initial concentrations for acetone, I2, and H+ and then you will measure the initial rate that results. From a practical standpoint, this method is typically very difficult as it requires one to quickly measure the initial rate of the reaction. We will simplify this considerably by manipulating conditions so that the rate of reaction is constant, thus removing the need to rush. Here’s how: As it happens, this reaction is 0th order with respect to I2. We will use a small quantity of I2 and relatively huge excesses of acetone and H+; I2 will be the limiting reactant, so the [acetone] and [H+] will not change appreciably as the tiny amount of I2 is consumed. Since the only concentration that will change significantly is [I2], and since the rate of reaction is 0th order in I2, then the rate of the reaction will not change. We can therefore find the average rate over the entire course of reaction, and that will be equal to the instantaneous initial rate. The rate of the reaction for each trial can be easily calculated as rate = -Δ[I2] = [I2]initial Δt t Procedure Part I – Determining x, y, z, and k You will run each reaction in a large test tube, and in each reaction you will have a total of 50.0 mL of liquid. In the first trial you will combine 10 mL of each reagent with 20 mL of water, being careful to add the I2 last, at which point you will swirl vigorously and begin timing. In subsequent trials you will change one of the reagent concentrations (by doubling its volume), while keeping the other two concentrations the same. Add water as necessary so the total volume is always 50.0 mL. volume (mL) of 4 M Acetone volume (mL) of 1 M HCl volume (mL) of 0.005 M I2 volume (mL) of water I 10.0 10.0 10.0 20.0 II 20.0 Trial III IV 20.0 20.0 Time (sec) 1st Run Time (sec) 2nd Run Temp (ºC) Obtain approximately 50 mL of each reagent solution in a small, covered beaker. You will also need a supply of fresh DI water. Combine the acetone, HCl, and water in a small Erlenmeyer flask, in the amounts listed in the table above. Note the time, add the I2 solution, swirl vigorously, and transfer the contents of the flask to one of the large test tubes. The reaction is complete when the colored I2 is entirely consumed. In order to better judge when the I2 has been completely consumed, you will compare the color of the reaction mix to a standard. In the first trial, the standard will be another large test tube filled to the same level with distilled water. In each successive trial, the standard will be the leftover reaction mix from the previous trial. Place a sheet of white paper on the bench beneath both test tubes and monito the color from above, looking down the length of each tube. In the table above, record the temperature of the reaction mixture and the time required for the I2 to be consumed. Repeat each trial. The results should agree within 20 seconds. Part II – The effect of temperature on rate Pick one of the reaction mixtures you used in Part I (not the slowest trial), and repeat the same reaction mix at approximately 0ºC and 40ºC. Record the Temps and times for all three trials in the table below. Reaction mixture used: Temperature (ºC) Time (sec) Part III (time permitting) – Using the rate law to make a prediction Create a reaction mix that is different from any you used in Part I. Use the completed rate law to predict rate of the reaction, then use the rate to predict the time of the reaction. Run the reaction and record the actual time of reaction. volume (mL) of 4 M Acetone volume (mL) of 1 M HCl volume (mL) of 0.005 M I2 volume (mL) of water Predicted Rate Predicted Time (sec) Actual Time (sec) Data and Calculations Part I - Dilution calculation: [diluted] = [original] x (volume of part)/(total volume) Mixture I II III IV [Acetone], (M) [I2], (M) [H+], (M) Rate = [I2]/time Part I - Determination of orders: By comparing Trials I and II, we can determine the order with respect to Acetone. You will write the rate law using the data for Trial I, then write the rate law using the data from Trial II, then divide Rate II by Rate I: Rate II = Rate I x = order in Acetone = Choose appropriate Trials to determine the other two orders. Show your work here. Rate Rate = y = order in I2 = Rate Rate = x = order in H+ = Part I - Determination of k: Now that you know the orders for each reactant, plug the concentrations and orders for each trial into the rate law and calculate k. Mixture I II III IV Average k Part II – Determination of Eact: Use linear regression and the Arrhenius’ Equation to determine the Eact of the reaction. Arrhenius’ Equation: (k1/k2) = - Eact/R (1/T1 – 1/T2) slope = -Eact/R Temperature (K) Rate Results of linear regression: slope = Eact = k ln k r= 1 T Prelab summary Procedural Tips: Add I2 last. Note time when it’s added, and swirl vigorously in Erlenmeyer. Compare reaction mix color to standard (DI water in Trial I, leftover reaction mix in other trials) Keep reactants cover. Acetone and I2 are volatile. Do one trial with each reaction mixture first. Do repeat trials if time permits. Safety: Acetone is flammable. Disposal: Pour all reagents in the large waste beaker in the fume hood. Prelab Questions A chemist studying the iodination of acetone performed two trials with the following mixtures: volume (mL) of 4 M Acetone volume (mL) of 1 M HCl volume (mL) of 0.005 M I2 volume (mL) of water Time (sec) I 10.0 10.0 10.0 20.0 250 II 20.0 10.0 10.0 10.0 120 Trial (a) Find the concentration of each reactant in Mixture I. Show your work. [acetone] = [I2] = [H+] = (b) Calculate the rate of the reaction in each Trial. Show your work. Rate I = Rate II = (c) Write the Rate Law for each Trial, plugging in values for all concentrations and for the rate. Rate II = Rate I = Divide Rate II by Rate I to determine the order with respect to acetone. Show additional work below. order =