Standard 1 – Chemistry Review
advertisement

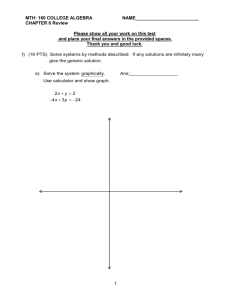
Standard 1 – Chemistry Review Name: _____________________ Score: _____________ DNM _____ Meets _______ Exceeds_______ Atoms, Elements, Molecules, Compounds, and Mixtures Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Sodium is an element found in table salt. It contains 11 protons and 12 neutrons. How many electrons are found in a neutral atom of sodium? a. 11 c. 23 b. 12 d. 1 ____ 2. Soil is made up of pieces of rocks, wood, decayed plants, and many other materials. Soil is an example of a. an atom. c. a compound. b. an element. d. a mixture. ____ 3. A pure substance that can be separated into two or more simpler substances by chemical means is a. an element. c. a formula. b. a compound. d. an atom. ____ 4. A molecule of carbon dioxide is shown above. Based on the makeup of the molecule, what is the correct chemical formula for carbon dioxide. a. CO b. 2CO c. C2O d. CO2 ____ 5. The chemical formula for glucose is C6H12O6. How many different elements make up one molecule of glucose? a. 1 c. 12 b. 3 d. 24 ____ 6. A material has a definite volume but a changing shape. What is it? a. a liquid c. a solid b. a gas d. ice ____ 7. The melting and boiling points of silver are respectively 960C and 1950C. What is its freezing point? a. 0C c. 960C b. 100C d. 1950C ____ 8. As a substance is changing from liquid to a gas, the distance between its molecules a. increases, and the temperature of the system remains the same. b. increases, and the temperature of the system increases. ____ c. decreases, and the temperature of the system remains the same. d. decreases, and the temperature of the system decreases. 9. Which substance has a definite shape and a definite volume? a. a crystal of table salt c. water vapor b. a solution of saltwater d. iced tea ____ 10. Which of the following is an example of a chemical change? a. water evaporating c. ice cream melting b. carbon joining with oxygen to form d. a pencil breaking carbon dioxide ____ 11. What happens during a physical change? a. New substances are formed. b. The identity of the substance is changed. c. A chemical reaction occurs. d. A substance may look different but not lose its identity. ____ 12. What two quantities must be known to calculate the density of a sample of matter? a. color and mass c. length and mass b. mass and volume d. solubility and mass ____ 13. Which physical property would be the same as a substance’s melting point? a. boiling point c. freezing point b. specific heat d. solubility ____ 14. Which of the following is the basis for arranging the elements in the modern periodic table? a. alphabetical order c. number of protons b. masses of atoms d. date of discovery ____ 15. How many elements are listed in the modern periodic table? a. 28 c. 72 b. 60 d. more than 100 ____ 16. What do elements in the same column have in common? a. similar chemical properties c. masses of their atoms b. number of protons d. size of their atoms ____ 17. Element Oxygen Carbon Gold Calcium Atomic Number 8 6 79 40 Which element in the table above is listed first on the periodic table? a. oxygen c. gold b. carbon d. calcium ____ 18. What does the law of conservation of matter state? a. The total mass of the reactants is greater c. The total mass of the reactants equals the than the total mass of the products. total mass of the products. b. The total mass of the reactants is less than d. Matter cannot change form. the total mass of the products. ____ 19. Na2O + H2O ? a. 1 b. 2 c. 3 d. 4 ____ 20. 16g ? CH4 + O2 2CO2 + H2O 44g 36g During a chemical reaction, methane (CH4) and oxygen (O2) combine to produce carbon dioxide (CO2) and water (H2O). The balanced chemical equation identifies the reactants and products for this reaction along with some of their masses. What mass (in grams) of oxygen (O2) is required for this reaction to occur? a. 16 b. 36 c. 44 d. 64 RCT Review - 1 swer Section ULTIPLE CHOICE 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: A D B D B A C B A B D B C C D A B C B D PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1