Vibratome cryostat SOP - University of Rhode Island
advertisement

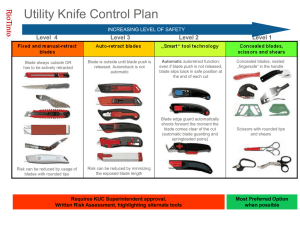
RHODE ISLAND GENOMICS and SEQUENCING CENTER Standard Operating Procedure VIBRATOME UltraPro 5000 Cryostat SAFETY INFORMATION • All users must submit a Sample Identification Form to the manager for approval before use in the RIGSC. • All users sectioning BSL-2 material must read and comply with the Vibratome BSL-2 SOP. Microtome Knives/Blades: Microtome knives and blades can be hazardous in the laboratory. • DO NOT leave the microtome unattended with an exposed blade in position. Remove the blade or cover it with the guards provided. • DO NOT leave blades lying on the bench top. DISPOSE of used blades in the sharps container. • DO NOT place used blades in the wastebasket. • DO NOT carry blades around with you. • DO NOT clean the blade along its length. Wipe the blade from the back edge to the cutting edge. • REMOVE the blade when you have finished using the cryostat. DO NOT leave a used blade in the blade holder of the cryostat. DISPOSE of the used blade in the sharps container. • REMEMBER used blades are dangerous - they are still sharp and may have been used to cut potentially infectious specimens. Operation: • When placing an object holder in the microtome or when orienting, manipulating or in any way placing your fingers in a position above the edge of the blade - ensure that the handwheel is locked and the knife guards are in position. Low Temperatures: • Parts of this instrument may attain temperatures as low as minus 50° C. Avoid touching any cold surfaces with bare skin. Wear glove liners and Nitrile gloves when using the specimen quick freezer or the microtome. Avoid touching the knife blade - it is cold as well as sharp. Always use knife guards when not sectioning. Rev.: 10/25/2012 Vibratome cryostat SOP 1 RHODE ISLAND GENOMICS and SEQUENCING CENTER INSTRUCTIONS 1. The cryostat power is normally left ON to maintain the chamber temperature. Standard settings: -23° C for Chamber Temperature; -10° C for Specimen Temperature. Adjust the temperatures as necessary for your personal protocol. 2. Turn ON specimen Quick Freezer (QF), 1 hr before use. Ensure that the appropriate sleeve is in place for the object holder to be used. To reduce frost, keep the QF cover in place when not freezing specimens. SAFETY: The OF operates at extremely low temperatures and may cause freeze burns to the user. Wear glove liners and Nitrile gloves when using the specimen quick freezer or the microtome. 3. At time of use, remove the insulated night plug, close window and turn the light ON. 4. Make sure that knife block is at its rear position by releasing the clamp lever and turning coarse advance counter-clockwise until it stops. 5. Remove a new blade from the package and carefully clean with absolute alcohol and a Kimwipe. Insert blade in the blade holder, position edge for cutting specimen, and tighten blade clamp lever. Allow blade to equilibrate to chamber temperature for at least 15 min. before use. 6. When QF temperature has reached -80° C, it is ready to be used. At room temperature, apply a layer of embedding medium to the top of an object holder and place in QF. As soon as the medium starts to turn white, place specimen on top and quickly add a layer of embedding medium to cover the specimen. Avoid trapping bubbles in the medium. Allow specimen to freeze completely before removing the object holder. 5. Remove the object holder from the QF and place it in the jaws of the vise on the microtome and tighten. Wait 15 min. for the object holder to equilibrate to the specimen temperature before trimming. 6. Trim the specimen. Turn the handwheel to bring the object holder with specimen opposite the level of the edge of the knife. Release the knife block clamp lever and turn the coarse advance control to bring the knife-edge close to the specimen. Turn the coarse advance in small increments while rotating the handwheel to trim off thin layers of the frozen medium. Continue trimming until the specimen is exposed to the point desired for harvesting thin/thick sections. Lock the knife block clamp lever. 7. Set the section thickness control to the desired position. Rotate the handwheel to cut thin or thick sections. When the first sections are cut, lower the anti-roll plate into position. When making a change in section thickness, first turn control back to zero, then increase setting to the desired thickness. 8. To collect sections, bring a clean microscope slide at room temperature (RT) towards the knife, swing out the anti-roll plate and move the slide just above the section(s). The section(s) should jump and stick to the slide. Remove slide to RT when not collecting sections. When slide is filled, allow to air dry at RT before staining. Keep the knife and specimen free of debris by brushing upwards with a small paintbrush. Rev.: 10/25/2012 Vibratome cryostat SOP 2 RHODE ISLAND GENOMICS and SEQUENCING CENTER SHUT DOWN Upon completion of sectioning: • Turn OFF specimen Quick Freezer. LEAVE MAIN POWER ON. • Position handwheel with object holder at top of travel and LOCK the handwheel. • Turn section thickness control to ZERO and press rewind button. If the auto-rewind does not work, turn the black handle on the top of the microtome to rewind. • Remove feather blade from knife holder and discard in sharps box. • Remove object holder, place in beaker in sink. Remove specimen with running warm water. • Release knife block clamp lever and turn coarse control counterclockwise to bring the stage completely back. • Remove and cleanup as much sectioning debris as possible using a Kimwipe and 95% alcohol. Discard wipes in the trash. • Slide window up, turn off light, and replace insulated night plug. Rev.: 10/25/2012 Vibratome cryostat SOP 3