Aldehydes and ketones
advertisement
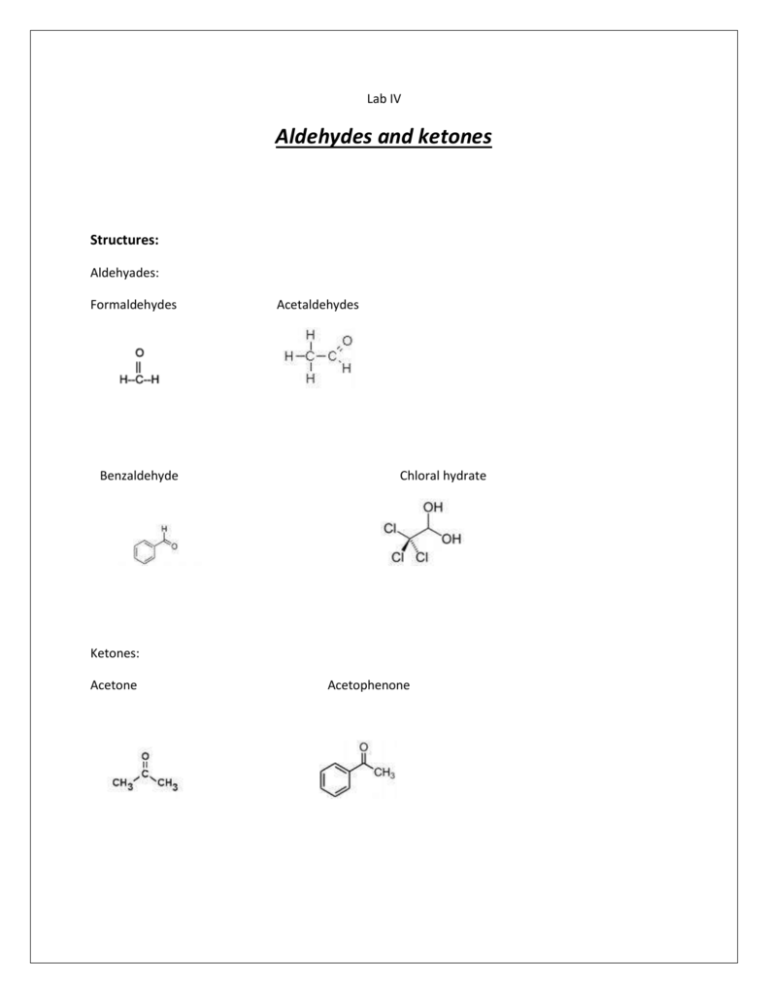
Lab IV Aldehydes and ketones Structures: Aldehyades: Formaldehydes Benzaldehyde Acetaldehydes Chloral hydrate Ketones: Acetone Acetophenone I-Physical properties: State: Formaldehydes: liquid, mobile, true liquid. Acetaldehydes: Benzaldehyde: Chloral hydrate: solid Acetone: Acetophenone : Color: Colorless except chloral hydrate which is white in color. Odor 1. 2. 3. 4. 5. 6. Formaldehyde :Pungent odor Acetaldehyde: Benzaldehyde: bitter almond odor. Chloral hydrate: Acetone : characteristic odor of ketones . Acetophenone: Ignition test : 1. Inflammability : Formaldehyde & acetaldehyde : Benzaldehyde : Chloral hydrate : non inflammable, Acetone & acetophenon : 2. 3. 4. 5. Change in appearance : Color imparted to the flame: Odor of gas evolved : Residue : Comment : II- tests for Acid- base properties (solubility or miscibility) Formaldehyde, acetaldehyde, acetone and chloral hydrate: miscible in water. Effect on L.P : NO CHANGE . Benazaldehyde : immiscible in water ( oil droplet ) COMMENT : III-Preliminary general chemical tests: 1. Soda lime : on cold Few granules soda lime+ few mg of chloral hydrate chloroform odor ( sweaty odor) 2. 30 % NAOH: formaldehyde acetaldehyde benzaldehyde Chloral hydrate Acetone acetophenon ON COLD No reaction Yellow resin ON HOT Cannizzaro’s reaction No reaction Chloroform odor +oil droplet No reaction No reaction Cannizzaro’s reaction comment Dilute it with water(aldol condensation) No reaction No reaction Cannizzaroe’s reaction: self oxidation reduction reaction 3. Fecl3 : no reaction either on cold or hot. 4. Conc. H2SO4: Few mg. chloral hydrate + 2 drops conc. H2SO4 Shaking separation and the formation of Chloral layer IV-Class general test: 1-2,4-Dinitrophenylhydrazine test ( brady’s reagent ) Reagent used: 1-Reagent A 2- Reagent B aldehydes and ketone miscible in water aldehydes and ketones immiscible in water. Reaction : 1 ml of aldehyde or ketone + 3 drops of reagent A or B shaking Results: Formaldehydes : Acetaldehyde : Chloral hydrate: Acetone : yellow ppt. orange ppt. no reaction. orange ppt. Benzaldehyde : orange ppt. Acetophenone : orange ppt. 2- Silver mirror test ( tollens’ reagent test) or ammoniacal silver nitrate test : Reaction : 1- 5 mls of silver nitrate + 1 drop 10% NAOH Black ppt. hydroxide(drop by drop ) shaking clear solution . add dilute ammonium 2- add 1 ml of aldehyde or ketone + 1 ml of tollens’ reagent heat on W.B. ( 1O\ ) Cool on the rack. Results: Formaldehyde: rapidly gives silver mirror. Acetaldehyde: rapidly gives silver mirror. Benzaldehyde: silver mirror after longer time on the water bath. Chloral hydrate: silver mirror after longer time on the water bath. Acetone acetophenone: -v reaction. 3- Fehling reagent: Reaction: 1-2 mls of fehling A solution + 2 mls of fehling B solution fehling reagent ( blue color 2- 1 ml of aldehyde or keton + 1 ml of 10% NAOH + 1 ml of fehlling reagent RESULTS: Any chang of the blue color to red is a +v result. Formaldehyde , acetaldehyde : rapidaly gives +v reslut. Chloral hydrate, benzaldehyde : takes longer time to give +v results. Aceton , acetophenon : -v reaction. on W.B FOR 10\ v- specific tests : 1- Aldehydes: a- formaldehyde & acetaldehyde : Test steps Na-nitroprusside 1ml of aldehyde+3 drops of Na- nitroprusside + 1 ml of 10 % NaoH acetaldehyde Red color formaldehyde -v Iodoform 1 ml of aldehyde (diluted with water) +1 ml 10% NaoH + excess I2 shake & wait 5\ Yellow resin -v Salicylic acid 2 drops of aldehyde + few mg of salicylic acid+2 -V drops of conc. H2SO4 on the wall of T.T Crimson red color on the wall of the T.T b- Chloral hydrate: 1- conc. H2SO4. 2- Soda lime test. 3- 30% NaoH. C- Benzaldehyde : - auto-oxidation test : on a watch glass add few drops Benzaldehyde leave it for 20\ crystals of benzoic acid. 2-Ketones: TEST Iodoform steps As before Na- nitroprusside metadinitrobenzen As before 1 ml of keton+ few mg of metadinitrobenzen + 1 ml 10% NaoH Acetone Yellow precipitant Red color Violet color rapidly disappear N.B: acetophenone should first be dissolved in ethanol or methanol. Acetophenone Yellow precipitant Red color Persistent Violet color