Media Release
advertisement

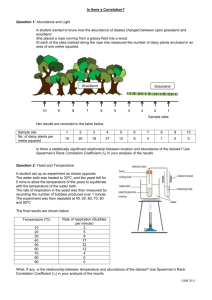
Laying the foundation for yeast-based opioid synthesis *IMAGES* NATURE CHEMICAL BIOLOGY Chemical biology Embargo London: Monday 18 May 2015 16:00 (BST) New York: Monday 18 May 2015 11:00 (EDT) Tokyo: Tuesday 19 May 2015 00:00 (JST) Sydney: Tuesday 19 May 2015 01:00 (AEST) An engineered yeast that can complete the early steps of opioid synthesis, producing (S)reticuline from glucose, is reported in a paper published online this week in Nature Chemical Biology. Previous studies have shown that engineered yeasts can complete the final steps of opioid synthesis. Future research to refine and bridge these pathways may eventually allow for large-scale, low cost production of opioids. Many, widely-used drugs are isolated or manufactured from plant extracts because their structural complexity precludes their cost-effective synthesis in the laboratory. Systems using engineered microbes, such as yeasts, to produce these compounds are recently becoming a reality thanks to advances in DNA sequencing and synthetic biology. Benzylisoquinoline alkaloids (BIAs) are a large family of plant-derived chemicals that include the compounds morphine and codeine. They have been difficult to produce using microbes because a key enzyme early in the pathway that can work in yeast to convert L-tyrosine to L-DOPA (a precursor to dopamine), has not been found. To address this, John Dueber and colleagues developed a unique colour-coded biosensor which allowed them to identify the missing enzyme, which they then mutated to make it more productive. They genetically engineered the yeast Saccharomyces cerevisiae to produce this enzyme, allowing the first demonstration of the conversion of glucose to dopamine by yeast. The authors then modified the yeast further, adding DNA from other species, so that it could perform subsequent reactions in the pathway, eventually producing the intermediary, (S)-reticuline. One more step is now required to bridge the two pathways. Pamela Peralta-Yahya states in an accompanying News & Views that “Given that downstream BIA pathway enzymes have already been shown to express in yeast, this work opens the door to the production of complex BIAs directly from glucose.” Article and author details 1. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose Corresponding Author John Dueber, Berkeley University of California Berkeley, Berkeley, California, United States Email: jdueber@berkeley.edu, Tel: +1 510 643 4616 News & Views Author Pamela Peralta-Yahya, Atlanta Georgia Institute of Technology, Atlanta, Georgia, United States Email: pperalta-yahya@chemistry.gatech.edu DOI 10.1038/nchembio.1816 Online paper* http://nature.com/articles/doi:10.1038/nchembio.1816 * Please link to the article in online versions of your report (the URL will go live after the embargo ends). Geographical listings of authors Canada & United States Image 1 Caption: Yeast cells producing the yellow beet pigment betaxanthin, which UC Berkeley researchers used to quickly identify key enzymes in the production of benzylisoquinoline alkaloids (BIAs), the metabolites in the poppy plant that could lead to morphine, antibiotics and other pharmaceutical agents. Credit: William DeLoache at UC Berkeley