Animal Ethics Committee - University of Canberra
advertisement

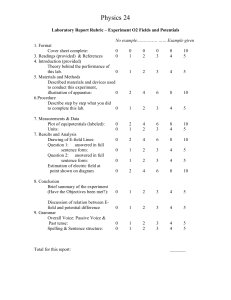
Animal Ethics Committee Application for the Use of Animals in Teaching, Laboratory and Field Research The information sought in this form is required under the ACT Animal Welfare Act (1992), the NSW Animal Research Act and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes NHMRC, 8th Edition (2013). All investigators must have read the requirements of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, NHMRC, 8th Edition (2013), before completing this application. The Code is available at: http://www.nhmrc.gov.au/guidelines/publications/ea28. Numbers in square brackets refer to the relevant parts of the Code. The application must provide clear evidence of the steps taken to comply with the general principles for the care and use of animals for scientific purposes by addressing the issues of: the replacement of animals with other methods the reduction in the number of animals used; and the refinement of techniques to reduce the impact on animals. To fill out this form, type your answer in the space provided. Where there is a choice (e.g. Yes or No), place an (X) in the box indicating your choice. If there is insufficient space for your answer, please expand existing space and repaginate the form when complete. The application must stand alone, any references to proposed techniques or procedures not currently in the Standard Operating Procedures: for the care and use of animals in laboratory/ teaching and field research (located at http://www.canberra.edu.au/ucresearch/ethics/animal) need to be explained in detail. Please forward the signed application to the Research Ethics & Compliance Officer by the closing date for papers. A hard copy is no longer required if the electronic copy of the application includes all relevant signatures. Research Ethics & Compliance Officer Animal Ethics Committee Secretariat animalethicscommittee@canberra.edu.au Student investigators please note: Where a project involves a student proceeding to a higher degree, the student’s supervisor must be listed as the chief investigator at Question 1 and 38. (Revised August 2013) PART 1 SUMMARY OF APPLICATION 1. Project title: [2.4] 2. Expected date of commencement and Start date: duration of the data collection: [2.4] 3. Applicant’s name: Title Given names End date: Surname Email address Investigator names: Note: A Student cannot be the sole investigator on a project. Please refer above regarding student investigators. Chief investigator Co-investigator Co-investigator 4. The project is: Research based (X) Laboratory based (X) Teaching (X) Field based (X) 5. The project is for: PhD (X) Other: Hons. (X) 6. Please identify all the locations at which research using animals will be conducted. Full street addresses should be supplied where applicable. Location 1: Location 2: Location 3: After initial approval of a project, two (2) annual renewals are permitted before a new project application is required. Projects must be renewed every 12 months. An Annual Review form will need to be completed for each renewal and at the completion of the project. 7. The purpose of the project. [2.7.4] Which of the following best describes the purpose of the project? (Mark one or multiple (X)) (a) Research into the basic mechanisms of life (b) Research into human health problems (c) Environmental/biological research (d) Livestock, health and animal production (e) Development of a commercial product (f) Batch and diagnostic testing (g) Observational Studies (h) Teaching primary (i) Teaching secondary (j) Teaching tertiary (k) Native and introduced wildlife research PART 2 ABOUT THE PROJECT 8. Please answer questions 8a – f below by placing an (X) against the appropriate answer [1.1 [iii] and 1.10-1.14]] a) Do the procedures for the project: Require animals to be killed without prior experimentation, before the preparation of whole animal or tissue specimens? (X) Yes No (Revised August 2013) b) If you answered Yes to a), please go to Question 9. If you answered No to question a), please continue. Require anaesthesia and the animal(s) to be killed after the procedures without Regaining consciousness? (X) Require anaesthesia and the recovery of the animal(s) for subsequent investigation? (X) Have a possibility of causing pain or distress to the animals? (X) Involve long-term restraint of animals or multiple surgical interventions? (X) Involve (X) a) Raising antisera b) Behavioural studies Yes No Has the project been the subject of a previous application? (X) Yes No If you answered Yes, please quote the reference number: CEAE Number 10. a) Institution involvement other than University of Canberra: [2.6.4] Will any part of this project be carried out at another institution? (X) Yes No b) Has approval been sought from the institution's ethics committee? (X) Yes No c) d) e) f) c) Ionising radiation d) Recombinant DNA/RNA e) Carcinogens or teratogens 9. If you answered Yes, please name the institution and the project reference number. Institution Name: Ref. No.: 11. a) Outline in language intelligible to a non-scientist the following: [2.4.12] Aim of the project: b) All procedures to be employed in carrying out the project: Provide sufficient detail for an informed layperson to understand the procedures. Identify all factors and procedures that may have an impact on the animal’s well-being. This may include handling, housing, etc as well as specific experimental procedures. This also includes details on any invasive, confining or observational procedure that may affect, directly or indirectly, the natural movement or behaviour of the animal. Details should further include treatment substance names, dose rates, routes of administration, etc (if relevant specify the animal’s body size and/or weight; indicate whether anaesthetic and analgesic are used but provide details in your answer Question 31 and 32). Where UC approved Standard Operating Procedures (SOP) exist, refer to the SOP numbers instead of providing detailed descriptions. If any proposed procedures differ from the approved SOP, please detail all proposed changes. (Revised August 2013) 12. Please outline the potential value of the project in obtaining or establishing significant information relevant to one or more of the following: [2.7.4 (ii)] a) the understanding of humans or animals; b) the maintenance and improvement of human or animal health and welfare; c) the improvement of animal management or production; d) achievement of educational objectives. 13. Please list alternatives you have considered to replace the use of animals in this project and explain why each alternative is unsuitable? [2.7.4 (iii)] For further information please go to http://www.canberra.edu.au/ucresearch/ethics/animal/alternatives-toanimal-testing 14. Does the project pose any health risks or hazards to other animals or staff? Yes No [2.4.25] If you answered Yes, please list the risks and outline what procedures will be used to minimise those risks? If this project is being conducted for the purposes of RESEARCH, please go to PART 3 If this project is being conducted for the purposes of TEACHING, please go to PART 4 PART 3 15. RESEARCH PROJECTS (ONLY) Is the project part of an application to a funding body? (X) Yes No If you answered Yes, please attach a copy of the application to the funding body. In the case of large grant applications such as those typically required for ARC or NHMRC grants, please append ONLY the covering page of the grant application and the section of the application containing the project description (i.e. Section E for ARC applications) 16. Does this project repeat previously performed projects? (X) Yes No If you answered Yes, why is it necessary to repeat the project? (Revised August 2013) PART 4 TEACHING PROJECTS ( ONLY ) 17. Unit Name: Year level of course: 18. Estimated number of students undertaking the unit during the semester: 19. Is the project compulsory for students? (X) [4.12] Yes No If you answered Yes, how and when were the students made aware of the requirement for this type of project? 20. Would students be disadvantaged if the project/procedure was not conducted? (X) [4.12] Yes No 21. Will the students be handling live animals? (X) [4.13-4.14] Yes No If you answered Yes, please explain what steps have been or will be taken to ensure that the students are trained in animal handling techniques, and adequately supervised while handling the animals: PART 5 22. ABOUT THE ANIMALS Please complete either of the tables below for the species which are to be used in the project. [2.7.4 (vii) FIELD SURVEY WORK (ONLY) If this work is being undertaken as part of a field survey or monitoring program, please indicate numbers or estimated number of animals expected in the 1st Year: Species name (in Latin) Common name Total number (if known) OR LABORATORY EXPERIMENTS (ONLY) If this work is being undertaken as part of a laboratory experiment please indicate numbers or estimated number of animals expected in the 1st Year: Species name (in Latin) Strain Number required Source of supply (Revised August 2013) 23. Year Year 2 Year 3 How many of each species will be required in each of the subsequent years of the project? [2.7.4 (ix)] Species name (in Latin) Strain/ common name Number required a) Are permits for the use and/ or transport of these animals required? (X) Yes No [2.7.4 (xxii)] List relevant permits and authorising body? Please append copies of the relevant permits. 25. a) Numbers of animals. [2.7.4 (ix)] Why is it necessary to use this number of animals? b) Has advice been sought from a statistician? (X) c) What statistical analysis has been undertaken to ensure that the proposed project design will show significant results or achieve educational objectives, using the minimum necessary number of animals? 26. a) Animal housing and management. [3.1] Where will animals be housed? b) Describe the type of housing to be provided: c) What will be the maximum and minimum number of animals per cage/ pen? 27. If animals are to be killed, describe the methods of killing, and what steps you will take to ensure that they are killed humanely? [3.3.45] 28. What arrangements will be made for the disposal of animals or animal tissues at the completion of the project? [2.4.21 – 2.4.24] a) Will the animals or animal tissues be made available to other researchers? Yes No [2.7.4 (x)] If you answered No, please explain why the animals or animal tissues will not be made available. 24. b) Yes No (Revised August 2013) If you answered Yes, please provide details of where the animals or animal tissues will be stored and name(s) of researcher involved. c) If you answered YES to Question 8a (Part 2) “animals to be killed without prior experimentation” please go to Part 6 If you answered NO to Question 8a (Part 2) “animals to be killed without prior experimentation” please continue to Question 29 29. Is the project likely to cause pain or distress to the animals? [2.7.4 (xiv) & (xv)] Please refer to the definitions of “Pain” and “Distress” in the Code If you answered Yes, please provide a justification for conducting the experiment: Yes No 30. Will the animals die as a result of the conditions of the project, or as an end point to the project? (X) [1.13] If you answered Yes, please provide a justification for conducting the project: Yes No 31. Yes No Please provide the following details of the experimental techniques: [2.7.4 (xiv)] Will anaesthesia or analgesia be used? (X) Yes No 32. a) Do factors exist that affect the health of animals and determine the endpoint of the project (e.g. tumour size, maximum weight loss)? If you answered Yes, please provide details: Name of anaesthetic/analgesic or tranquillising agent and dose (mg/kg body weight) Agent Dose (mg/kg body weight) b) Please describe the method that you will use to ensure that the anaesthesia is adequate: c) What surgical or other procedures will be used: 33. Will neuromuscular and similar blocking agents be used? [3.3.14] Yes No If you answered Yes, please supply full details of the monitoring procedures used to ensure that the potentially painful nature of the procedure is blocked by appropriate anaesthesia and analgesia: (Revised August 2013) 34. If anaesthesia or analgesia cannot be used and the animal may experience pain, please provide a full rationale for not using either: [3.3.8 – 3.3.15] 35. What procedures will be used to monitor animals during the project? [2.7.4 (xv)] 36. What procedures will be used to monitor the animals at the conclusion of the project? [2.7.4(xv)] 37. Have any of these animals been the subjects of previous projects? (X) a) b) c) No If you answered Yes, please provide details on: Previous project number Which animals? Brief outline of the previous project d) Why is it necessary to re-use the animals in this project? PART 6 38. Yes ABOUT THE APPLICANT(S) Name of Applicant and/or Chief Investigator [2.4] Name Institution/ Division /Discipline Room No. Telephone No. CEAE Authorisation (X) Yes No 1 If you answer No, please complete and attach an Authorisation to Conduct Experiments using Animals form. All individuals must be authorised before they can be involved in the project. Qualifications: Field of expertise: (Revised August 2013) All investigators should be listed and, if CEAE Authorisation has not been granted, it should be applied for. If you answer No, please complete an Authorisation to Conduct Experiments Using Animals form. All individuals must be authorised before they can be involved in the project (add additional rows, if more Co investigators or others directly associated with use of animals in the project are involved). 39. Co-investigator directly associated with the use of animals in the project Name Institution/ Division /Discipline Room No. Telephone No. CEAE Authorisation (X) Yes No 1 Qualifications: Field of expertise: 40. Co-investigator directly associated with the use of animals in the project Name Institution/ Division /Discipline Room No. Telephone No. CEAE Authorisation (X) Yes No 1 Qualifications: Field of expertise: 41. Co-investigator directly associated with the use of animals in the project Name Institution/ Division /Discipline Room No. Telephone No. CEAE Authorisation (X) Yes No 1 Qualifications: Field of expertise: 42. Name Nominee in charge of animals when Chief Investigator is absent: Telephone (Work) Telephone (Home) 43. Who will be in charge for the management of emergencies; provide name(s) and contact details. How will you ensure that the nominee(s) can be contacted? Nominee(s) Phone Email 1 2 44. Are any of the investigators students at the University of Canberra? Yes No (Revised August 2013) If you answered Yes, please provide the following information: Student name Student ID No. Name of supervisor 1 2 Note: A student cannot be a sole investigator PART 7 DECLARATION BY INVESTIGATOR(S) & DEPARTMENT HEAD(S) I/We: have read the current Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, NHMRC 8th Edition 2013 and agree to comply with the requirements of the Code and all relevant Commonwealth, State and Territory legislation, and am/are licensed or authorised as necessary to perform experiments using animals in the locations where the project will be conducted, and have checked and approved that where animals are to be held, the appropriate facilities are available for the species and that the facilities meet the standards as outlined in Section 4, acquisition and care of animals in breeding and holding facilities, of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Signatures: Applicant and/ or Chief Investigator Co-investigator Co-investigator Co-investigator Co-investigator Print name: Signatures: Print name: Signatures: Print name: Statement by Head of Faculty (Dean or delegate, e.g. Associate Dean Research or Director of Faculty Research Centre) / Director of University Research Centre I have read this application and adequate resources are available to undertake the project. I certify that the qualifications and experience of the investigators are appropriate to the procedures to be performed in accordance with this application. Print Name: Signature: Date: (Revised August 2013)