Response to reviewers` comments Reviewer #1 1. The description of
Response to reviewers’ comments
Reviewer #1
1. The description of prognostic value of SDC-1 should be changed (e.g. the title of the paper).
The title was changed to ‘Clinical implications in the shift of Syndecan-1 expression from the cell membrane to the cytoplasm in bladder cancer’
2. How is the 8 benign tissue characterized, which patients do the tissue originate from and how were they diagnosed?
The eight benign bladder paraffin blocks were from autopsy cases in which there was no record of hematuria or tobacco use. Median age was 26 years. Four were from males and four were from females.
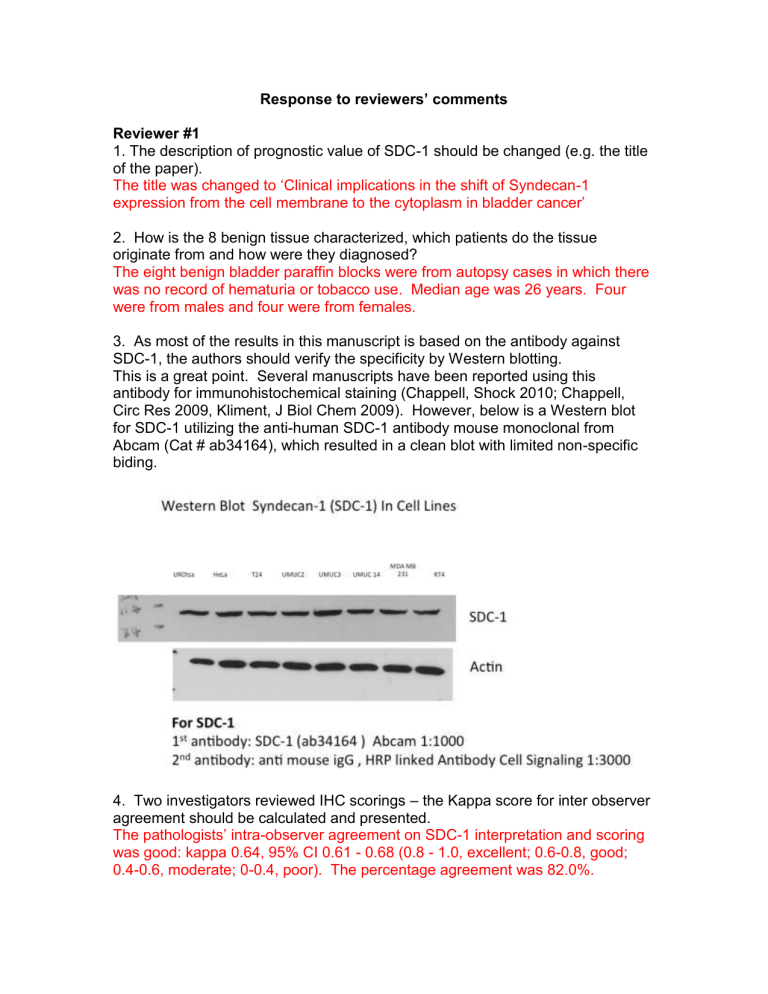
3. As most of the results in this manuscript is based on the antibody against
SDC-1, the authors should verify the specificity by Western blotting.
This is a great point. Several manuscripts have been reported using this antibody for immunohistochemical staining (Chappell, Shock 2010; Chappell,
Circ Res 2009, Kliment, J Biol Chem 2009). However, below is a Western blot for SDC-1 utilizing the anti-human SDC-1 antibody mouse monoclonal from
Abcam (Cat # ab34164), which resulted in a clean blot with limited non-specific biding.
4. Two investigators reviewed IHC scorings
– the Kappa score for inter observer agreement should be calculated and presented.
The pathologists’ intra-observer agreement on SDC-1 interpretation and scoring was good: kappa 0.64, 95% CI 0.61 - 0.68 (0.8 - 1.0, excellent; 0.6-0.8, good;
0.4-0.6, moderate; 0-0.4, poor). The percentage agreement was 82.0%.
5. The authors should present the details of the multivariate analysis. Which parameters were included etc. The 95% CI presented describes a huge range of the HR – were assumptions of proportional hazards verified before analysis?
Table 2 was added which describes the parameters included in the multivariate analysis.
6. Some language correction is needed on page 5: “Paraffin blocks…” and the following two sentences.
The language was revised.
Reviewer #2
1. It would be appropriate to analyse the levels of SDC-1 in the urine samples of the subjects used in the immunohistochemical study.
Yes ideally it would be great to have both urine and tissue from the same patient.
However in our tissue bank, which we utilized to conducted our urinary biomarker discovery and validation projects (Urquidi, BMC Urol 2012; Urquidi, Urology
2012; Urquidi, PLoSONE 2012; Urquidi, J Urol 2012; Goodison, PLoSONE 2012;
Rosser, J Urol 2013; Miyake, BMC Urol 2013), we only have access to urinary samples. Furthermore the archived IHC had longer follow-up, which helped when assessing survival. Going forward, we have collected urine and tissue samples (<50 subjects) and hope to use these samples in a follow-up study.
2. In addition, the RT-PCR and quantitative western blotting should be performed to quantify the production of mRNA and the protein level of SDC-1 respectively by bladder tissue.
As mentioned above, based on these encouraging initial results we have started to collect tissue and urine samples prospectively from bladder cancer patients to perform more detailed experiments in the near future. Note
– we did attempt to extract RNA from FFPE however found the quality (not quantity) of the RNA to be subpar to perform RT-PCR.
3. These experiments will clarify if the cancer bladder tissues (low-grade and
NMIBC) really produce more SDC-1 than the other tissues analysed in this study and if the SDC-1 in the urine really come from by bladder tissues.
Please note that we are not stating non-aggressive bladder tissue has more
SDC_1 than aggressive bladder cancer, but that the expression of the protein is shifted within the tissue. This is all that we can comment on at this time.
When we set out to perform this study we did not expect to see this shift in SDC-
1 expression from low-grade to high-grade and from low stage to high stage.
However as we stated above, now we are in the process of collecting tissue prospectively to confirm these findings. In the meanwhile, we are further exploring the biology behind SDC-1 in tissue culture. As is evident in these preliminary findings (CONFIDENTIAL), we have confirmed our clinical findings that more aggressive disease has reduced shed SDC-1, probably due to reduced
SDC-1 cell membrane expression. This will serve as a nidus for our follow-up study.
However since this is the first time the shift in SDC-1 expression has been reported in the bladder literature as well as the first time of assessing urinary protein levels of SDC-1, we believe this study is noteworthy and warrants publication. Furthermore at best, minimal SDC-1 immunoreactivity was not noted in the stroma of bladder tissue. Previously we reported a poor correlation of
SDC-1 with urinary blood (0.037) (Urquidi, BMC Urology 2012). Lastly due to its size, SDC-1 is not known to be excreted in urine. Thus based on our IHC, we believe SDC-1 is coming from the bladder tissue.
3. As the SDC-1 is a transmembrane heparan sulfate proteoglycan with three different domains (extracellular, transmembrane and cytoplasmic domains) which of these has been detected by ELISA assay? This explanation is necessary to support the conclusions of the authors.
Please note that the urine samples studied were processed to remove cellular content. We believe freezing whole urine then thawing would lead to lysis of
these cells and a misinterpretation of urinary proteins. Thus it is unlikely that the shed SDC-1 would be from transmembrane or cytoplasmic domain. The commercial ELISA kit utilized (Cat# ab46507 Abcam, Cambridge, MA, USA) does not report the epitope that it binds with. However this is an interesting question and related experiments will be incorporated into the above in vitro assays to further study SDC-1 in human bladder cancer.