Transplant Data and Sobi Collaboration
advertisement

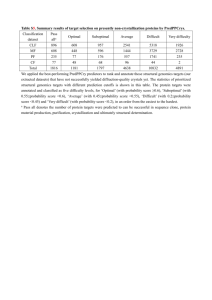
FOR IMMEDIATE RELEASE INFORM GENOMICS ANNOUNCES RESULTS OF STUDY PREDICTING RISK OF ORAL MUCOSITIS IN PATIENTS UNDERGOING HIGH DOSE CHEMOTHERAPY --Data to be presented at 2012 ASCO Annual Meeting---Collaboration agreement with Swedish Orphan Biovitrum established to further develop and commercialize the personalized medicine product-Boston, MA- April 18, 2012- Inform Genomics, Inc., a private company focused on developing novel platforms of personalized medicine products for cancer supportive care and inflammatory diseases, today announced the completion of the first phase of product development to predict a patient’s risk of developing oral mucositis after receiving high dose chemotherapy prior to hematopoietic stem cell transplant. The results of this single center, 153-patient study demonstrated the product’s ability to discriminate which patients develop oral mucositis with 99.3% accuracy and an area under the Receiver Operator Characteristic (ROC) curve of 99.7%. Further development will include validation of these initial results in a multicenter study. In addition, Inform Genomics announced that it entered into a collaboration agreement with Swedish Orphan Biovitrum AB (Sobi) to further develop and commercialize the product. Sobi is a leading integrated biopharmaceutical company dedicated to bringing innovative therapies and services to improve the health of rare disease patients and their families. “We are very pleased with the exciting results of this study,” said Ed Rubenstein, M.D., President & CEO of Inform Genomics, “and our agreement with Sobi demonstrates the value our technology can bring to biopharma partners while expanding the market opportunity for both companies’ products. When commercialized, this product will be available for the hematology oncology stem cell transplant market and will complement the target market of our lead product, OnPART™ for patients with solid tumors.” The principal investigator for the study, Stephen T. Sonis, D.M.D., D.M.Sc., Chief Scientific Officer of Biomodels, LLC, who also serves as the Chief of the Division of Oral Medicine at the Dana-Farber Cancer Institute and Professor of Oral Medicine at the Harvard School of Dental Medicine, will present the results of the study at the upcoming 2012 American Society of Clinical Oncology (ASCO) Annual Meeting, as part of the educational session titled “Mucosal Injury in Patients with Cancer: Targeting the Biology,” taking place from 11:30 am to 12:45 pm on Sunday, June 3, 2012 in Chicago, IL. About OnPART™ OnPART™, Oncology Preferences And Risk of Toxicity, will be Inform Genomics’ first platform molecular diagnostic test for personalizing treatment decisions for patients undergoing chemotherapy for colorectal, breast, lung or ovarian cancer. Based upon response rates and survival, more than one chemotherapy regimen may be considered appropriate care for patients with these common solid tumors, yet the regimens vary widely in their toxicity profiles, including nausea & vomiting, diarrhea, oral mucositis, cognitive dysfunction, fatigue and peripheral neuropathy. OnPART™ is being developed to assess genomic risk for these side effects, and to provide valuable information for patients and medical oncologists to help clarify clinical choices. 101 Federal Street Suite 1900 Boston, Massachusetts 02110 About Inform Genomics Inform Genomics, Inc. is a private company focused on developing novel platforms of personalized medicine products for cancer supportive care and inflammatory diseases, including its lead product, OnPART™, designed to predict an individual’s risk of six common toxicities of commonly used chemotherapy regimens based on his or her individual genomic profile. The Company’s business model leverages existing technology in conjunction with proprietary analytic methods for conducting genomewide association studies. Product development programs will lead to commercial, single source laboratory tests consisting of single-nucleotide polymorphism (SNP) clusters that determine the likelihood of individual patient clinical outcomes to drug therapies. The U.S. market opportunity for these differentiated products exceeds $2 billion annually. Inform Genomics is headquartered in Boston, Massachusetts. For more information, please visit www.informgenomics.com. Contact: Bill Brown, Chief Financial Officer bbrown@informgenomics.com 101 Federal Street Suite 1900 Boston, Massachusetts 02110
![9_Komlenac - start [kondor.etf.rs]](http://s2.studylib.net/store/data/005352037_1-bdc91b0717c49a75493200bca431c59c-300x300.png)