Supplementary materials - Springer Static Content Server
advertisement

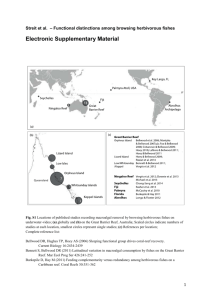
Supplementary materials (a) (b) 150°E 145°E N N 15°S Pioneer Bay 1 2 Cairns Orpheus Island Hazard Bay Townsville 0 20°S 150 300 km 0 1 2 km Figure S1: Map of the Great Barrier Reef (GBR) showing the location of the study sites. (a) Geographic location of Orpheus Island in the central GBR (b) Map of Orpheus Island showing the location of the study sites on its leeward margin. Filled circles represent the two study sites. Loffler, Bellwood and Hoey 2014 Galaxaura (n=20) Proportion of pairing instances Acanthophora (n=21) 0.6 0.6 0.5 0.5 0.4 0.4 0.3 0.3 0.2 0.2 0.1 0.1 0.0 0.0 Sargassum (n=26) Turbinaria (n=20) 0.6 0.6 0.5 0.5 0.4 0.4 0.3 0.3 0.2 0.2 0.1 0.1 0.0 0.0 m ra ra ria lgae lone ho xau ssu ina A p a oa a rg Turb acr tho Gal a n S a rm Ac he t O e e a a a o r a u r s s u m na r i ga l lon h a i x A p a o b a o cr rg Tur th G a l Sa an ma c r A he Ot Figure S2: Frequency of naturally occurring associations between macroalgal species on the reef within Pioneer Bay, Orpheus Island. Values are the proportion of instances Acanthophora, Galaxaura, Sargassum, and Turbinaria thalli were found in close proximity to other macroalgae or alone. Loffler, Bellwood and Hoey 2014 Table S1: Relative palatability of each species of macroalgae used in the study Algal species Taxonomic classification Functional classification (Steneck 1988) Defence Acanthophora spicifera Rhodophyta Corticated None Galaxaura rugosa Rhodophyta Calcified Calcified, chemical Turbinaria ornata Phaeophyceae Leathery Leathery Sargassum sp. Phaeophyceae Leathery Leathery, chemical Palatability References Readily consumed in macroalgal feeding trials Calcification and allelochemicals dissuade predation Tough, leathery morphology, making predation by many fish species difficult A dominant macroalgal species after the occurrence of phase shifts. Readily eaten in feeding trials Littler et al. 1986; Reinthal and Macintyre 1994 Paul and Hay 1986; Rasher et al. 2011; Rasher et al. 2013 Littler et al. 1983 Hughes et al. 2007; Fox and Bellwood 2008; Cvitanovic and Bellwood 2009 Loffler, Bellwood and Hoey 2014 Methods and results for macroalgal association surveys The frequency of associations among macroalgal species on the study reef was quantified visually. A minimum of 20 individuals of each of the four focal macroalgal species, Acanthophora spicifera, Galaxaura rugosa, Turbinaria ornata and Sargassum sp., were located while conducting a haphazard swim across the mid-outer reef flat, approximately 2m deep. Due to their size, algae greater than 3cm from the focal thalli would have been unlikely to provide or be provided refuge. Therefore, if no algae were within 3 cm of the focal alga, it was classified as 'alone'. These visual surveys revealed that macroalgae at our study sites are almost always found in close association with other algae and that the three pairings selected for this study are common among algal taxa on the reef of Pioneer Bay. Of the 20 Galaxaura thalli surveyed, 50 % were associated with Acanthophora (ESM Fig. S2). Acanthophora was predominantly associated with Sargassum (43 %), and was less often associated with Turbinaria (5%). Of the 26 Sargassum thalli surveyed, most were associated with 'other' macroalgae, such as Padina sp. and Halimeda sp., (50%), and Turbinaria (15%). Associations with Turbinaria were evenly spread with both Sargassum (25 %) and Acanthophora (25 %) being frequently recorded in its vicinity. The four focal species of algae were rarely found growing in isolation, with only one Acanthophora and two Turbinaria thalli observed without other macroalgae in their immediate vicinity. References Cvitanovic C, Bellwood D (2009) Local variation in herbivore feeding activity on an inshore reef of the Great Barrier Reef. Coral Reefs 28:127-133 Loffler, Bellwood and Hoey 2014 Fox RJ, Bellwood DR (2008) Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 27:605-615 Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory and the resilience of coral reefs to climate change. Curr Biol 17:360-365 Littler MM, Taylor, PR, Littler DS (1983) Algal resistance to herbivory on a Caribbean barrier reef. Coral Reefs 2:111-118 Littler MM, Taylor PR, Littler DS (1986) Plant defense associations in the marine environment. Coral Reefs 5:63-71 Paul VJ, Hay ME (1986) Seaweed susceptibility to herbivory: chemical and morphological correlates. Mar Ecol Prog Ser 33:255-264 Rasher DB, Hoey AS, Hay ME (2013) Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 94:1347-1358 Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME (2011) Macroalgal terpenes function as allelopathic agents against reef corals. Proc Natl Acad Sci U S A 108:17726-17731 Reinthal PN, Macintyre IG (1994) Spatial and temporal variations in grazing pressure by herbivorous fishes: Tobacco Reef, Belize. Atoll Res Bull 425:1-11 Loffler, Bellwood and Hoey 2014