Single Agent, 2 Weekly Cetuximab
advertisement
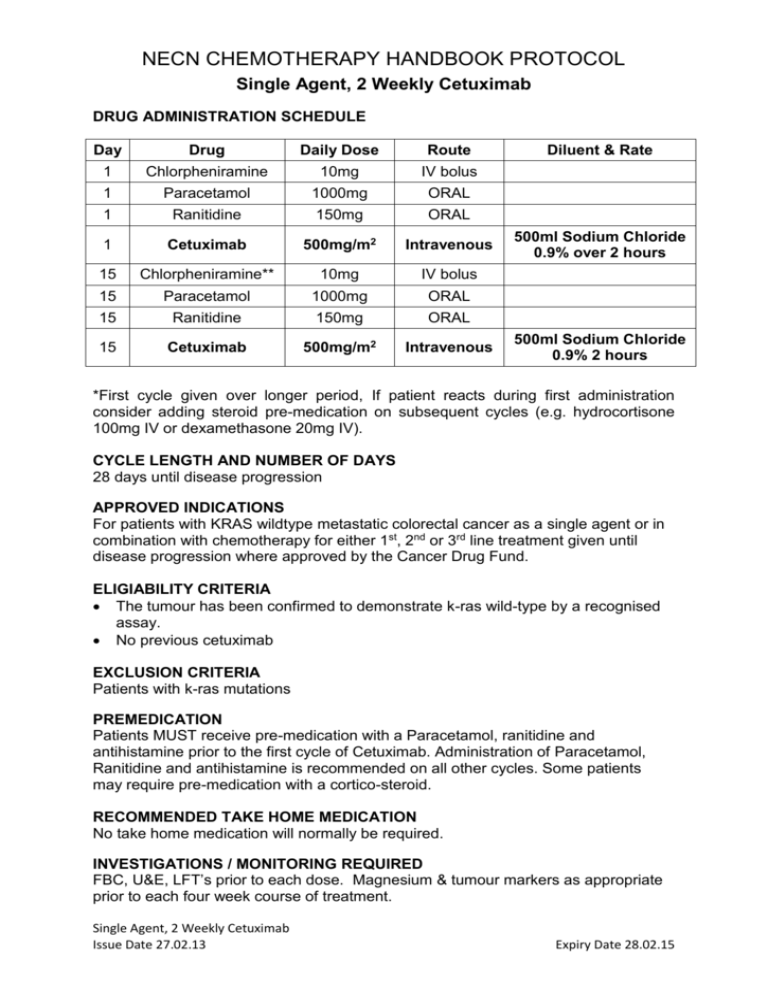
NECN CHEMOTHERAPY HANDBOOK PROTOCOL Single Agent, 2 Weekly Cetuximab DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route 1 Chlorpheniramine 10mg IV bolus 1 Paracetamol 1000mg ORAL 1 Ranitidine 150mg ORAL 1 Cetuximab 500mg/m2 Intravenous 15 Chlorpheniramine** 10mg IV bolus 15 Paracetamol 1000mg ORAL 15 Ranitidine 150mg ORAL 15 Cetuximab 500mg/m2 Intravenous Diluent & Rate 500ml Sodium Chloride 0.9% over 2 hours 500ml Sodium Chloride 0.9% 2 hours *First cycle given over longer period, If patient reacts during first administration consider adding steroid pre-medication on subsequent cycles (e.g. hydrocortisone 100mg IV or dexamethasone 20mg IV). CYCLE LENGTH AND NUMBER OF DAYS 28 days until disease progression APPROVED INDICATIONS For patients with KRAS wildtype metastatic colorectal cancer as a single agent or in combination with chemotherapy for either 1st, 2nd or 3rd line treatment given until disease progression where approved by the Cancer Drug Fund. ELIGIABILITY CRITERIA The tumour has been confirmed to demonstrate k-ras wild-type by a recognised assay. No previous cetuximab EXCLUSION CRITERIA Patients with k-ras mutations PREMEDICATION Patients MUST receive pre-medication with a Paracetamol, ranitidine and antihistamine prior to the first cycle of Cetuximab. Administration of Paracetamol, Ranitidine and antihistamine is recommended on all other cycles. Some patients may require pre-medication with a cortico-steroid. RECOMMENDED TAKE HOME MEDICATION No take home medication will normally be required. INVESTIGATIONS / MONITORING REQUIRED FBC, U&E, LFT’s prior to each dose. Magnesium & tumour markers as appropriate prior to each four week course of treatment. Single Agent, 2 Weekly Cetuximab Issue Date 27.02.13 Expiry Date 28.02.15 NECN CHEMOTHERAPY HANDBOOK PROTOCOL Single Agent, 2 Weekly Cetuximab Where CEA is elevated this should be measured before each cycle (no need to await result before proceeding with treatment). ASSESSMENT OF RESPONSE Tumour size and patient symptomatic response to be assessed at appropriate intervals. All patients must have radiological assessment of disease no later than 8 weeks after starting Cetuximab and treatment must be discontinued if there is evidence of disease progression. REVIEW BY CLINICIAN To be reviewed by either a Nurse, Pharmacist or Clinician before every cycle. NURSE / PHARMACIST LED REVIEW On cycles where not seen by clinician. ADMINISTRATION NOTES Cetuximab 500mg/m2 can be administered faster than 2 hours provided the infusion rate does not exceed 10mg/min. Cetuximab 2 weekly schedule is unlicensed therefore used with prescriber accepting responsibility for any drug reactions. Sodium chloride 0.9% must be used for line flushing with Cetuximab. Cetuximab (as with all monoclonal antibodies) can cause infusion reactions. Administration must only take place in facilities with resuscitation facilities. Management of Cetuximab infusion reactions is outlined in the table below: CTC Grade Allergic / Infusion Related Reaction Grade 1 – mild (Transient rash during infusion, drug fever < 38°C) Grade 2 – moderate (Rash during infusion, flushing, urticaria, dyspnoea, drug fever ≥ 38°C) Grade 3 or 4 – severe or life threatening (Symptomatic bronchospasm, with or without urticaria, parenteral medication indicated, allergy-related oedema, angiooedema, hypotension, anaphylaxis) Action required Reduce Cetuximab infusion rate by 50% and monitor for worsening of symptoms. Ensure infusion rate does not exceed 240mins (4hours). Use lower rate on all subsequent infusions. Stop Cetuximab infusion. Resume at 50% of rate once symptoms have resolved or reduced to Grade 1. Continue to monitor closely for worsening of symptoms. Use lower rate on all subsequent infusions. Stop infusion immediately. Do not re-treat with Cetuximab • Patients should be warned that on rare occasions they may experience an infusion reaction several hours after cetuximab – they should be advised to seek urgent medical help if this occurs. Single Agent, 2 Weekly Cetuximab Issue Date 27.02.13 Expiry Date 28.02.15 NECN CHEMOTHERAPY HANDBOOK PROTOCOL Single Agent, 2 Weekly Cetuximab Pulse, Respiration, Blood Pressure and Temperature must be measured during and 1 hour following infusion. TOXICITIES • Allergic reaction • Cardio-pulmonary arrest • Rash / skin reaction to Cetuximab • Hypomagnesia • Pulmonary toxicity DOSE MODIFICATION / TREATMENT DELAYS Haematological toxicity: Cetuximab does not normally cause myelosuppression, but is associated with anaemia in up to 10% of patients. Renal Function: Cetuximab is not renally excreted Skin reactions: CTC (v2) definition Delay Cetuximab (other chemotherapy can continue) Treatment Grade 1 Macular or popular eruption/erythema without symptoms No Grade 2 Macular or popular eruption/erythema with pruritis or other symptoms; localised desquamation or other lesions covering <50% of body No Grade 3 Symptomatic generalised erythroderma or macular, popular or vesicular eruption or desquamation covering >50% of body Topical anti-acne cream (eg benzoyl peroxide) for face. Salicylic acid in alcoholic lotion for chest/back As grade 1 plus As grade 2 plus menthol in aqueous saline compresses if cream. Oral required. antihistamine and oral tetracycline (for 3 months) Yes – see below Systemic or topical steroids for treatment of rash are not generally advised. Patients on tetracyclines should be advised to avoid prolonged exposure to sun. Topical treatments can have a drying effect on the skin. Care should be taken to avoid aggravating xerosis, especially when acne-like rash is fading or becoming scaly. Switch to moisturising creams instead of alcoholic lotion or gel if this occurs. Single Agent, 2 Weekly Cetuximab Issue Date 27.02.13 Expiry Date 28.02.15 NECN CHEMOTHERAPY HANDBOOK PROTOCOL Single Agent, 2 Weekly Cetuximab Occurrence of Grade 3 Toxicity First Second Third Fourth Dose once resolved to Grade 2 or better 250mg/m2 200mg/m2 150mg/m2 Discontinue treatment If skin toxicity has not resolved to Grade 2 or better within 3 weeks discontinue cetuximab TREATMENT LOCATION Can be given at Cancer Centre or Cancer Unit. Not suitable for home administration. REFERENCES: Tabernero J et al.,Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic / pharmacodynamic phase I dose-escalation study. Ann OncoL; 21: 1537 – 1545 (2010) Yuan Y et al.: Activity and tolerance of biweekly CapeOx-Cetuximab in first-line therapy of metastatic colorectal cancer (mCRC) and K-RAS influences. ASCO GI Cancers Symposium 2009 Abstract #341 Bouchahda M et al.: Feasibility of cetuximab given with a simplified schedule every 2 weeks in advanced colorectal cancer: a multicentre, retrospective analysis Med Oncol 28: S253-S258 (2011) Roca J-M, Alonso V, Pericay C et al.: Cetuximab given every 2 weeks plus irinotecan is an active and safe option for previously treated patients with metastatic colorectal cancer. Chemotherapy 56, 142-146 (2010) Martin-Martorell P et al.: Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: results of a phase II single institution trial. BJC 99: 455-458 (2008) Jensen BV et al.: Cetuximab every second week with irinotecan in patients with metastatic colorectal cancer refractory to 5-FU, oxaliplatin and irinotecan: KRAS mutation status and efficacy. Journal of Clinical Oncology 28 (Suppl.): Abstract 3573 1. Document Title: 2 weekly Cetuximab for mCRC Document No: CRP13U012 Author: Approved by: Due for Review: Current Version: Steve Williamson Consultant Pharmacist NECN Approval Signature* Calum Polwart, Network Pharmacist Date Approved: 1. 22.02.2013 Feb 2015 Summary of Changes Single Agent, 2 Weekly Cetuximab Issue Date 27.02.13 Expiry Date 28.02.15