(a) (b) (c)
advertisement

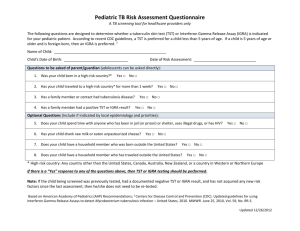
Tunneling effects in Bimolecular Nucleophilic Substitution (SN2) Reactions Wan-Chen Tsai and Wei-Ping Hu* Department of Chemistry and Biochemistry, National Chung Cheng University, Chia-Yi, Taiwan 621 Abstract Tunneling effects on the bimolecular rate constants of three SN2 reactions have been studied theoretically using dual-level dynamics approach with variational transition state theory including multidimensional tunneling. The three reactions, (1) gas-phase CN + CH3F, (2) gas-phase OH(H2O) + CH3F microsolvated reaction, and (3) OH + CHF in bulk water solvent represented models of S2 reactions with positive energy barrier heights and with various degrees of solvation. The calculated results indicated that the tunneling effects cannot be ignored in evaluating the reaction rate constants of the S2 reactions with even modest barrier heights (> 5 kcal/mol) at room temperature, and they contribute significantly to the rate constants at lower temperature. The calculation also suggested that, regardless of the levels of solvation, the tunneling effects resulted mostly from the motion of central carbon atom, not from the primary hydrogen atoms, through the sharp energy barriers. The heavy atom tunneling contributed importantly to the calculated carbon kinetic isotope effects (KIEs) at low temperature, and the 13C and 14C KIEs as large as 7 and 40 have been predicted. Introduction The ion-molecule nucleophilic substitution (SN2) reactions, which usually involve an alkyl halide and an anion (the nucleophilie), are ubiquitous and very important in organic and interstellar chemistry.13 Most methyl and ethyl halides can only undergo the SN2 pathway. However, for i-propyl or higher branched alkyl halides, the SN2 and E2 reactions may be competitive with the same reactants and produce the same ionic products.4,12,14,16,1921 This makes it difficult to distinguish these two pathways by experiments, especially in the gas phase. Therefore, measurement of the bimolecular rate constants and kinetic isotope effects (KIEs) can be very useful for investigating the reaction mechanisms. In the last three decades, there have been significant amount of experimental and theoretical studies on gas-phase SN2 reactions. Due to the experimental techniques employed and the rate of molecular collisions, the gas-phase rate constants could be measured in the range of 109 to 1012 cm3 molecule s1. This translates to the ion-molecule reactions with no barriers to with barrier heights of ~5 kcal/mol. It has been known that SN2 reactions usually show lightly inverse deuterium KIEs (kH/kD ≈ 0.71.0),412,1517,2022 while the E2 reactions show strong normal deuterium KIEs at room temperature (kH/kD ≈ 26).4,12,13,16,18,20,21,23 The SN2 reactions often proceed much efficiently in the gas phase than the in the solution. Due to the strong ion-dipole interaction, most exoergic SN2 reactions have very low energy barriers in the gas phase. However, in the solution phase, the strong solvation effects usually raise the energy barriers significantly.21 The SN2 reactions in the gas phase with small or negligible energy barriers are very fast with bimolecular rate constants approaching that of those of ion-dipole collisions. Recently, there have been some experimental and theoretical studies on the microsolvated systems (added one or a few solvent molecules) in attempt to connect the gap of our understanding between the gas phase and solution phase reactions.5,8,9,11,13,17,18,22,23 For example, Truhlar have evaluated the microsolvated system of Cl(H2O)n (n = 02) + CH3Cl reactions.5 As the microsolvated H2O molecules increase, the barrier heights were raised from 3.1 kcal/mol (n = 0), 5.4 kcal/mol (n = 1) to 10.7 kcal/mol (n = 2). Simultaneously, the rate constants were predicted to be 2.36 × 1014 cm3 molecule1 s1 (n = 0), 6.38 × 1018 cm3 molecule1 s1 (n = 1), and 2.07 × 1020 cm3 molecule1 s1 (n = 1), respectively. The predicted KIEs due to the CD3 substitutions were 0.96, 0.95, and 0.93 for n = 0, 1, and 2, and the KIEs from the D2O substitutions were 1.04 and 1.26 for n = 1 and 2, respectively. According to the results, we could inference the number of the microsolvated H2O molecules had no significant effects on the deuterium KIEs. Scheme 1 revealed the potential energy surface of the common gas-phase and solution-phase SN2 reactions. In the gas-phase, ion and molecule formed an ion-dipole complex with ~ 20 kcal/mol energy released before the transition state. The ion-dipole complex had high internal energy so that the reaction could easy overcome the transition state and form another ion-dipole complex before it separated into the products. Therefore, most gas-phase SN2 reactions have low barrier heights and fast reaction rate constants. For the solution-phase SN2 reactions, the negative charges were usually much more localized in the reactants than in the transition states, the solvation effects were usually stronger on the reactants, and thus the energy barriers were significantly higher in polar solvent than in the gas phase.2427 For example, Tanaka had measured the rate constants of several SN2 reactions in the gas phase and in solution.24 For the CH3Br + Cl reaction, the rate constants were 2.1 × 1011 cm3 molecule1 s1 and 8.2 × 1027 cm3 molecule1 s1, respectively. The solvation energies significantly raised the barrier height and decreased the reaction rate constants. There may be some differences of the kinetic isotope effects in the gas phase and in solution. The comparison of the CN + CH3I in the gas phase and in solution has been reported.21 In the gas phase, the computational KIE due to the CD3 substitution was 0.83 is in excellent agreement with the experimental KIE of 0.84 at 298 K. In the solution phase, the experimental KIE from the CD3 substitution was 0.902 in the 40% CH3OH/60% DMSO (v/v) solution at 293 K. As the solvation effects raising the energy barriers of the SN2 reaction, the tunneling effects may dramatic influence the rate constants and KIEs at low temperature due to the motion of hydrogen and α-carbon atoms. In the past, the rate constants of the high-barrier SN2 reactions were too slow that can’t be measured experimentally, and most theoretical studies on the SN2 rate constants and KIEs were based on the transition state theory (TST). However, the TST theory was only feasible for the small barrier (4 ~ 3 kcal/mol) systems. For the high barrier cases, the tunneling contribution to the rate constants and KIEs needed to be calculated accurately. In this research, we performed theoretical studies on three exothermic SN2 reactions, (1) gas-phase CN + CH3F, (2) gas-phase OH(H2O) + CH3F microsolvated reaction, and (3) OH + CH3F in bulk water solvent, which have positive energy barriers. We used the variational transition state theory with multidimensional tunneling (VTST/MT)2833 correction to calculate the dynamics behaviors of reaction rate constants and KIEs from the various isotope substitutions over a wide range of temperature. The energetics, geometries, reaction rate constants, kinetic isotope effects, solvent kinetic isotope effects, and tunneling effects of reactions were discussed in this report. Computational Methods Electronic Structure Calculations. The geometry and vibrational frequencies of stationary points and transition states were calculated using the B3LYP34 hybrid functional with the 6-311+G(d,p)35,36 basis set and MP237 theory with the 6-311+G(d,p), aug-cc-pVDZ, and aug-cc-pVTZ38,39 basis sets. Single-point energies were calculated at the CCSD(T)40/aug-cc-pVTZ level. Additionally, MLSE(C1)-M06-2X41 and CCSD(T)/aug-cc-pVQZ single point energies were also calculated for the gas-phase CN + CH3F reaction. Bulk solvation in water was modeled using the polarizable continuum model (PCM)4244 for the OH + CH3F reaction. All the electronic structure calculations were performed using the Gaussian 09 program.45 Dual-level VTST/MT Dynamics Calculations. The dual-level46,47 variational transition state theory with multidimensional tunneling (VTST/MT) correction was used in the present study to calculate the thermal rate constants over the temperature range of 35 K to 600 K. The method requires a qualitatively correct “low-level” potential energy surface (PES), and a set of “high-level” geometry and energy data on the stationary points along the reaction path for the interpolated corrections to the low-level PES. We selected the MP2/aug-cc-pVDZ to calculate the low-level PES as required for the VTST/MT calculation. The reaction path was calculated from 6.5 to 5.5 bohrs for gas-phase CN + CH3F reaction, 5.5 to 5.5 bohrs for gas-phase OH(H2O) + CH3F reaction, and 9.0 to 5.0 bohrs for OH + CH3F in bulk water solvent reaction with a gradient step size of 0.006 bohr and a hessian step size of 0.03 bohr using the Page-McIver method48,49 in the mass-scaled coordinates with a scaling mass of 1 amu. Redundant internal coordinate systems5053 were used in the vibrational analysis along the reaction path. The interpolated correction was based on the ion-dipole complexes for the gas-phase CN + CH3F and OH(H2O) + CH3F reactions, and was based on the reactants and products for the OH + CH3F in bulk water solvent. The high-level geometries were obtained at the MP2/aug-cc-pVTZ level, and the high-level energies data were obtained at CCSD(T)/aug-cc-pVTZ (CCSD(T)/aug-cc-pVQZ for gas-phase CN + CH3F reaction. The SIL-1 interpolated correction scheme54 was applied in the dual-level calculation using the CCSD(T)/aug-cc-pVTZ energies along the low-level reaction path geometries to estimate the barrier widths. Thermal rate constants as a function of temperature were calculated at the conventional transition state theory (TST), canonical variational theory (CVT),28,29 canonical variational theory with small-curvature tunneling approximation (CVT/SCT),30,33,55 and canonical variational theory with microcanonical optimized multidimensional tunneling correction (CVT/μOMT)33 levels. Since the SCT tunneling probabilities were larger than LCT, the CVT/μOMT rate constants were identical to the CVT/SCT rate constants of these three SN2 reactions in this research. Thus, we only showed the CVT/SCT rate constants in the results. The rotation symmetry number of CH3F (C3v) is three. For CN + CH3F, the rotation symmetry number of transition state (C3v) is three, so the overall symmetry numbers of reaction were set to one. For OH(H2O) + CH3F, the transition state structure (C1) is chiral, so the overall symmetry numbers of reaction were set to six. For OH + CH3F, the symmetry of transition state is Cs, therefore the overall symmetry numbers of reaction were set to three. The dual-level VTST/MT calculations were performed using the Gaussrate 8.4H program, which is a locally modified version of Gaussrate 8.2 program56 to work with the new Gaussian 09 program. The Gaussrate program primarily served as an interface between the Gaussian 09 and Polyrate 8.2 programs.57 Results and Discussion Gas-phase CN + CH3F reaction (a) Geometry and Energetics Figure 1 shows the calculated geometries of the CN + CH3F → F + CH3CN reaction at the MP2/aug-cc-pVTZ level. References 1. Wladkiwski, B. D.; Wilbur, J. L.; Brauman, J. I. J. Am. Chem. Soc. 1994, 116, 2471. 2. Meng, Q.; Gogoll, A.; Thibblin, A. J. Am. Chem. Soc. 1997, 119, 1217. 3. Gronert, S.; Fagin, A. E.; Wong, L. J. Am. Chem. Soc. 2007, 129, 5330. 4. Gronert, S.; Depuy, C. H.; Bierbaum, V. M. J. Am. Chem. Soc. 1991, 113, 4009. 5. Zhao, X. G.; Tucker, S. C.; Truhlar, D. G. J. Am. Chem. Soc. 1991, 113, 826. 6. Viggiano, A. A.; Morris, R. A.; Paschkewitz, J. S.; Paulson, J. J. Am. Chem. Soc. 1992, 114, 10477. 7. Boyd, R. J.; Kim, C. K.; Shi, Z.; Weinberg, N.; Wolfe, S. J. Am. Chem. Soc. 1993, 115, 10147. 8. O’Hair, R. A. J.; Dang, T. T.; DePuy, C. H.; Bierbaum, V. M. J. Am. Chem. Soc. 1994, 116, 3609. 9. Hu, W.-P.; Truhlar, D. G. J. Am. Chem. Soc. 1994, 116, 7797. 10. Hu, W.-P.; Truhlar, D. G. J. Am. Chem. Soc. 1995, 117, 10726. 11. Viggiano, A. A.; Arnold, S. T.; Morris, R. A.; Ahrens, A. F.; Hierl, P. M. J. Phys. Chem. 1996, 100, 14397. 12. Hu, W.-P.; Truhlar, D. G. J. Am. Chem. Soc. 1996, 118, 860. 13. Wu, Y.-R.; Hu, W.-P. J. Am. Chem. Soc. 1999, 121, 10168. 14. Almerindo, G. I.; Pliego, R., Jr. Org. Lett. 2005, 7, 1821. 15. Fang, Y.-R.; MacMillar, S.; Eriksson, J.; Kołodziejska-Huben, M.; Dybała-Defratyka, A.; Paneth, P.; Matsson, O.; Westaway, K. C. J. Org. Chem. 2006, 71, 4742. 16. Villano, S. M.; Kato, S.; Bierbaum, V. M. J. Am. Chem. Soc. 2006, 128, 736. 17. Davico, G. E. J. Phys. Chem. A 2006, 110, 13112. 18. Eyet, N.; Villano, S. M.; Kato, S.; Bierbaum, V. M. J. Am. Soc. Mass Spectrom. 2007, 18, 1046. 19. Bento, A. P.; Solà, M.; Bickelhaupt, F. M. J. Chem. Theory Comput. 2008, 4, 929. 20. Villano, S. M.; Eyet, N.; Lineberger, W. C.; Bierbaum, V. M. J. Am. Chem. Soc. 2009, 131, 8227. 21. Garver, J. M.; Fang, Y.-R.; Eyet, N.; Villano, S. M.; Bierbaum, V. M.; Westaway, K. C. J. Am. Chem. Soc. 2010, 132, 3808. 22. Chen, J.-L.; Hu, W.-P. J. Chin. Chem. Soc. 2012, 59, 1401. 23. Eyet, N.; Villano, S. M.; Bierbaum, V. M. J. Phys. Chem. A 2013, 117, 1136. 24. Tanaka, K.; Mackay, G. I.; Payzant, J. D.; Bohme, D. K. Can. J. Chem. 1976, 54, 1643. 25. Chandrasekhar, J.; Smith, S. F.; Jorgensen, W. L. J. Am. Chem. Soc. 1984, 106, 3049. 26. Vayner, G.; Houk, K. N.; Jorgensen, W. L.; Brauman, J. I. J. Am. Chem. Soc. 2004, 126, 9054. 27. Reichardt, C.; Welton, T. Solvent Effects on the Rates of Homogeneous Chemical Reactions. In Solvents and Solvent Effects in Organic Chemistry, Fourth Edition; Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2010; pp. 165. 28. Truhlar, D. G.; Garrett, B. C. Acc. Chem. Res. 1980, 13, 440. 29. Truhlar, D. G.; Isaacson, A. D.; Garrett, B. C. In Theory of Chemical Reaction Dynamics; Baer, M., Ed.; CRC Press: Boca Raton, FL, USA, 1985; Volume 4, pp. 65. 30. Lu, D.-H.; Truong, T. N.; Melissas, V. S.; Lynch, G. C.; Liu, Y.-P.; Garrett, B. C.; Steckler, R.; Isaacson, A. D.; Rai, S. N.; Hancock, G. C.; Lauderdale, J. G.; Joseph, T.; Truhlar, D. G. Comput. Phys. Commun. 1992, 71, 235. 31. Truong, T. N.; Lu, D.-H.; Lynch, G. C.; Liu, Y.-P.; Melissas, V. S.; Gonzalez-Lafont, A.; Rai, S. N.; Steckler, R.; Garrett, B. C.; Joseph, T.; Truhlar, D. G. Comput. Phys. Commun. 1993, 75, 143. 32. Liu, Y.-P.; Lynch, G. C.; Truong, T. N.; Lu, D.-H.; Truhlar, D. G.; Garrett, B. C. J. Am. Chem. Soc. 1993, 115, 2408. 33. Liu, Y.-P.; Lu, D.-H.; Gonzalez-Lafont, A.; Truhlar, D. G.; Garrett, B. C. J. Am. Chem. Soc. 1993, 115, 7806. 34. Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623. 35. Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650. 36. Clark, T.; Chandrasrkhar, J.; Spitznagel, G. W. Schleyer, P. V. R. J. Comp. Chem. 1983, 4, 294. 37. Møller, C.; Plesset, M. S. Phys. Rev. 1934, 46, 618. 38. Dunning, T. H. J. Chem. Phys. 1989, 90, 1007. 39. Kendall, R. A.; Dunning, T. H.; Harrison, R. J. J. Chem. Phys. 1992, 96, 6796. 40. Pople, J. A.; HeadGordon, M.; Raghavachari, K. J. Chem. Phys. 1987, 87, 5968. 41. Sun, Y.-L.; Li, T.-H.; Chen, J.-L.; Hu, W.-P. Chem. Phys. Lett. 2009, 475, 141. 42. Miertus, S.; Scrocco, E.; Tomasi, J. Chem. Phys. 1981, 55, 117. 43. Miertus, S.; Tomasi, J. Chem. Phys. 1982, 65, 239. 44. Tomasi, J.; Mennucci, B.; Cammi, R. Chem. Rev. 2005, 105, 2999. 45. Gaussian 09, Revision A.02, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian, Inc., Wallingford CT, 2009. 46. Hu, W.-P.; Liu, Y.-P.; Truhlar, D. G. J. Chem. Soc. Faraday Trans. 1994, 90, 1715. 47. Corchado, J.C.; Espinosa-Garcia, J.; Hu, W.-P.; Rossi, I.; Truhlar, D. G. J. Phys. Chem. 1995, 99, 687. 48. Page, M.; McIver, J. W. J. Chem. Phys. 1988, 88, 922. 49. Page, M.; Doubleday, C.; Mclver, J. M. J. Chem. Phys. 1990, 93, 5634. 50. Pulay, P.; Fogarasi, G. J. Chem. Phys. 1992, 96, 2856. 51. Jackels, C. F.; Gu, Z.; Truhlar, D. G. J. Chem. Phys. 1995, 102, 3188. 52. Nguyen, K. A.; Jackels, C. F.; Truhlar, D. G. J. Chem. Phys. 1996, 104, 6491. 53. Chuang, Y.-Y.; Truhlar, D. G. J. Phys. Chem. A 1998, 102, 242. 54. Huang, C.-H.; You, R.-M.; Lian, P.-Y.; Hu, W.-P. J. Phys. Chem. A 2000, 104, 7200. 55. Zhao, X. G.; Lu, D.-H.; Liu, Y.-P.; Lynch, G. C.; Truhlar, D. G. J. Chem. Phys. 1992, 97, 6369. 56. Corchado, J. C.; Chunag, Y.-Y.; Coitino, E. L.; Truhlar, D. G. Gaussrate, version 8.2; University of Minnesota: Minneapolis, MN, USA, 1999. 57. Chuang, Y.-Y.; Corchado, J. C.; Fast, P. L.; Villa, J.; Hu, W.-P.; Liu, Y.-P.; Lynch, G. C.; Nguyen, K. A.; Jackels, C. F.; Gu, M. Z.; Rossi, I.; Coitino, E. L.; Clayton, S.; Melissas, V. S.; Steckler, R.; Garrett, B. C.; Isaacson, A. D.; Truhlar, D. G. Polyrate—Version 8.2; University of Minnesota: Minneapolis, MN, USA, 1999. Table 1. Calculated reaction energeticsa (in kcal/mol) of the gas-phase CN + CH3F → F + CH3CN reaction. Erxnb B3LYP/6-311+G(d,p) MP2/6-311+G(d,p) 3.2 5.2 MP2/aug-cc-pVDZ MP2/aug-cc-pVTZ 7.2 5.9 1.2 1.4 1.2 CCSD(T)/aug-cc-pVTZc CCSD(T)/aug-cc-pVQZc MLSE(C1)-M06-2Xc energies relative to CN + CH3F bEnergy of reaction cUsing MP2/aug-cc-pVTZ structures aAll ion-dipole complex ion-dipole complex barrier height CN...CH3F F…CH3CN 8.9 8.3 28.9 14.5 9.5 28.1 9.2 9.5 31.0 11.3 9.1 29.9 11.7 9.1 25.6 12.0 8.9 25.7 12.1 9.2 25.7 Table 2. Calculated rate constants (in cm3 molecule1 s1) by the TST and CVT/SCT methods of the gas-phase CN + CH3F reaction. CN + CH3F CN + CD3F CN + 13CH3F CN + 14CH3F CN + 13CD3F T(K) TST CVT/SCT TST CVT/SCT TST CVT/SCT TST CVT/SCT TST CVT/SCT 35 50 70 6.43(90)a 4.15(67) 6.36(52) 1.03(32) 7.79(33) 7.38(33) 3.13(89) 1.17(66) 1.24(51) 1.39(32) 1.05(32) 9.93(33) 4.17(90) 3.03(67) 5.02(52) 1.94(33) 1.48(33) 1.43(33) 2.86(90) 2.30(67) 4.09(52) 4.30(34) 3.32(34) 3.28(34) 1.91(89) 8.18(67) 9.58(52) 2.71(33) 2.07(33) 1.99(33) 90 100 150 200 250 300 400 1.73(43) 1.56(40) 1.22(31) 3.86(27) 2.15(24) 1.58(22) 4.01(20) 9.10(33) 1.15(32) 1.81(30) 1.07(26) 3.80(24) 2.27(22) 4.71(20) 2.77(43) 2.33(40) 1.50(31) 4.33(27) 2.31(24) 1.66(22) 4.17(20) 1.24(32) 1.55(32) 2.17(30) 1.19(26) 4.05(24) 2.37(22) 4.87(20) 1.43(43) 1.31(40) 1.08(31) 3.50(27) 1.98(24) 1.47(22) 3.79(20) 1.84(33) 2.41(33) 1.10(30) 8.82(27) 3.33(24) 2.05(22) 4.38(20) 1.21(43) 1.12(40) 9.65(32) 3.21(27) 1.84(24) 1.38(22) 3.61(20) 4.39(34) 6.01(34) 7.65(31) 7.56(27) 3.00(24) 1.88(22) 4.12(20) 2.25(43) 1.93(40) 1.31(31) 3.92(27) 2.12(24) 1.55(22) 3.94(20) 2.59(33) 3.38(33) 1.34(30) 9.84(27) 3.57(24) 2.15(22) 4.54(20) 500 1.29(18) 1.44(17) 1.38(18) 1.46(17) 1.34(18) 1.50(17) 1.43(18) 1.51(17) 1.23(18) 1.38(17) 1.30(18) 1.38(17) 1.18(18) 1.33(17) 1.24(18) 1.33(17) 1.28(18) 1.44(17) 1.35(18) 1.44(17) 600 a6.43(90) means 6.43 × 1090 Table 3. Calculated KIEs by the TST and CVT/SCT methods of the gas-phase CN + CH3F reaction. KIE(CD3) KIE(13CD3) KIE(13C) TST CVT/SCT KIE(14C) TST CVT/SCT TST CVT/SCT T(K) TST CVT/SCT 35 50 70 0.205 0.356 0.511 0.744 0.743 0.743 1.541 1.370 1.265 5.322 5.258 5.144 2.244 1.802 1.554 24.009 23.478 22.492 0.337 0.507 0.664 3.810 3.769 3.698 90 100 150 200 250 300 400 0.624 0.668 0.815 0.890 0.930 0.950 0.962 0.737 0.740 0.832 0.902 0.937 0.957 0.967 1.210 1.191 1.134 1.104 1.085 1.073 1.058 4.947 4.763 1.645 1.213 1.140 1.107 1.075 1.430 1.388 1.266 1.204 1.167 1.142 1.112 20.732 19.112 2.362 1.414 1.268 1.204 1.144 0.768 0.807 0.930 0.986 1.011 1.019 1.018 3.521 3.401 1.352 1.087 1.063 1.055 1.037 500 600 0.963 0.962 0.965 0.963 1.049 1.044 1.060 1.051 1.095 1.085 1.114 1.098 1.010 1.005 1.021 1.011 Table 4. Calculated reaction energeticsa (in kcal/mol) of the gas-phase OH(H2O) + CH3F reaction. F(H2O) + CH3OH Erxnb F + CH3OH(H2O) ion-dipole complex F + H2O + CH3OH OH(H2O)...CH3F F(H2O)...CH3OH barrier height 11.4 12.5 44.1 45.1 1.9 6.8 12.7 11.9 12.2 44.4 42.9 43.5 2.9 6.1 5.1 B3LYP/6-311+G(d,p) MP2/6-311+G(d,p) 21.3 22.1 1.3 7.3 5.3 MP2/aug-cc-pVDZ MP2/aug-cc-pVTZ 21.2 19.9 20.0 0.6 1.7 1.8 5.6 7.7 7.8 CCSD(T)/aug-cc-pVTZc energies relative to OH(H2O) + CH3F bEnergy of reaction cUsing MP2/aug-cc-pVTZ structures aAll 1.2 Table 5. Calculated rate constants (in cm3 molecule1 s1) by the TST and CVT/SCT methods of the gas-phase OH(H2O) + CH3F reaction. OH(H2O) + CH3F OH(H2O) + CD3F OD(H2O) + CH3F OH(D2O) + CH3F T(K) TST CVT/SCT TST CVT/SCT TST CVT/SCT TST CVT/SCT 35 50 1.31(59)a 7.35(46) 1.65(25) 1.20(25) 1.20(58) 3.05(45) 2.26(25) 1.71(25) 1.90(58) 4.49() 2.53(25) 1.84(25) 6.69(57) 5.55(44) 8.35(25) 6.18(25) 70 90 100 150 200 250 300 1.03(36) 1.24(31) 7.51(30) 1.79(24) 9.93(22) 4.90(20) 7.20(19) 1.14(25) 1.49(25) 1.96(25) 1.38(23) 2.36(21) 8.05(20) 9.91(19) 2.58(36) 2.37(31) 1.31(29) 2.40(24) 1.19(21) 5.52(20) 7.86(19) 1.69(25) 2.25(25) 2.99(25) 1.85(23) 2.82(21) 9.04(20) 1.08(18) 3.53(36) 3.08(31) 1.66(29) 2.82(24) 1.33(21) 5.98(20) 8.30(19) 1.75(25) 2.29(25) 3.04(25) 2.11(23) 3.17(21) 9.88(20) 1.15(18) 2.21(35) 1.33(30) 6.32(29) 7.36(24) 2.86(21) 1.14(19) 1.46(18) 5.99(25) 7.90(25) 1.04(24) 5.64(23) 6.86(21) 1.89(19) 2.03(18) 400 500 600 2.48(17) 2.90(17) 2.43(16) 2.64(16) 1.24(15) 1.30(15) OD(D2O) + CH3F 2.63(17) 3.07(17) 2.56(16) 2.78(16) 1.30(15) 1.36(15) OH(H2O) + 13CH3F 2.68(17) 3.16(17) 2.55(16) 2.79(16) 1.28(15) 1.35(15) OH(H2O) + 14CH3F 4.21(17) 4.96(17) 3.69(16) 4.04(16) 1.74(15) 1.83(15) OH(H2O) + 13CD3F T(K) TST CVT/SCT TST CVT/SCT TST CVT/SCT TST CVT/SCT 35 50 8.23(56) 3.02(43) 1.28(24) 9.36(25) 8.76(60) 5.48(46) 5.84(26) 4.30(26) 6.19(60) 4.24(46) 2.18(26) 1.62(26) 7.51(59) 2.18(45) 8.13(26) 6.21(26) 70 90 100 150 6.89(35) 3.04(30) 1.29(28) 1.08(23) 8.92(25) 1.17(24) 1.54(24) 8.06(23) 8.26(37) 1.04(31) 6.36(30) 1.59(24) 4.18(26) 5.72(26) 7.92(26) 9.99(24) 6.81(37) 8.86(32) 5.50(30) 1.43(24) 1.61(26) 2.32(26) 3.38(26) 7.61(24) 2.02(36) 1.95(31) 1.09(29) 2.12(24) 6.26(26) 8.76(26) 1.21(25) 1.33(23) 200 250 300 400 500 600 3.57(21) 1.31(19) 1.58(18) 4.27(17) 3.63(16) 1.68(15) a1.31(59) means 8.58(21) 2.17(19) 2.20(18) 5.06(17) 4.00(16) 1.78(15) 1.31 × 1059 9.03(22) 4.53(20) 6.73(19) 2.35(17) 2.32(16) 1.19(15) 2.01(21) 7.18(20) 9.06(19) 2.71(17) 2.51(16) 1.24(15) 8.31(22) 4.23(20) 6.35(19) 2.24(17) 2.23(16) 1.15(15) 1.75(21) 6.49(20) 8.37(19) 2.56(17) 2.39(16) 1.19(15) 1.08(21) 5.10(20) 7.34(19) 2.49(17) 2.44(16) 1.25(15) 2.39(21) 8.05(20) 9.85(19) 2.88(17) 2.64(16) 1.30(15) Table 6. Calculated KIEs by the TST and CVT/SCT methods of the gas-phase OH(H2O) + CH3F reaction. KIE(CD3) KIE(OD(D2O)) KIE(OD) TST CVT/SCT TST CVT/SCT TST CVT/SCT KIE(D2O) T(K) TST CVT/SCT 35 50 70 0.110 0.241 0.399 0.728 0.702 0.676 0.069 0.164 0.292 0.651 0.651 0.651 0.002 0.013 0.047 0.197 0.194 0.190 0.000 0.002 0.015 0.129 0.128 0.128 90 100 150 200 250 300 400 0.524 0.574 0.745 0.837 0.888 0.917 0.942 0.660 0.657 0.743 0.839 0.890 0.919 0.944 0.404 0.452 0.635 0.747 0.820 0.868 0.924 0.650 0.646 0.652 0.745 0.815 0.862 0.918 0.093 0.119 0.243 0.347 0.428 0.492 0.588 0.188 0.189 0.244 0.344 0.425 0.489 0.584 0.041 0.058 0.166 0.278 0.376 0.457 0.580 0.128 0.128 0.171 0.275 0.371 0.451 0.573 500 0.950 0.952 0.953 0.948 0.658 0.654 0.669 0.661 600 0.954 0.956 0.970 0.965 0.713 0.709 KIE(13CD3) 0.737 0.729 KIE(13C) KIE(14C) T(K) TST CVT/SCT TST CVT/SCT TST CVT/SCT 35 50 70 1.493 1.342 1.249 2.819 2.790 2.727 2.113 1.731 1.515 7.537 7.392 7.072 0.174 0.336 0.512 2.025 1.933 1.821 90 1.199 2.600 1.404 6.419 0.638 1.700 100 150 200 1.182 1.128 1.100 2.481 1.377 1.174 1.366 1.253 1.195 5.812 1.807 1.352 0.688 0.846 0.923 1.618 1.034 0.989 250 300 400 500 600 1.082 1.070 1.055 1.047 1.042 1.121 1.094 1.067 1.054 1.047 1.159 1.135 1.106 1.090 1.081 1.239 1.185 1.131 1.105 1.090 0.962 0.981 0.993 0.995 0.994 1.000 1.006 1.007 1.003 1.000 Table 7. Calculated reaction energeticsa (in kcal/mol) of the OH + CH3F → F + CH3OH reaction in bulk water solvent. B3LYP/6-311+G(d,p) MP2/6-311+G(d,p) MP2/aug-cc-pVDZ MP2/aug-cc-pVTZ CCSD(T)/aug-cc-pVTZb Erxnb 25.7 27.0 25.7 barrier height 24.3 24.2 16.9 16.2 13.3 19.0 14.6 to OH + CH3F in bulk water solvent. Solvation energies in water were calculated using the PCM model. bEnergy of reaction cUsing MP2/aug-cc-pVTZ structures. aRelative Table 8. Calculated reaction energeticsa (in kcal/mol) of the OH + CH3F → F + CH3OH reaction in bulk water solvent. Erxnb barrier height B3LYP/6-311+G(d,p) MP2/6-311+G(d,p) MP2/aug-cc-pVDZ 25.7 27.0 25.7 13.3 19.0 14.6 MP2/aug-cc-pVTZ 24.3 24.2 16.9 16.2 CCSD(T)/aug-cc-pVTZb to OH + CH3F bEnergy of reaction cUsing MP2/aug-cc-pVTZ structures aRelative Table 9. Calculated rate constants (in cm3 molecule1 s1) by the TST and CVT/SCT methods of the OH + CH3F reaction in bulk water solvent. OH + CH3F T(K) 35 50 TST CVT/SCT 1.11(119)a 6.80(45) 1.53(87) 1.24(44) 70 90 100 150 200 250 300 3.56(66) 2.49(54) 3.45(50) 9.12(38) 1.57(31) 9.31(28) 3.26(25) 400 500 600 5.65(22) 7.25(22) 5.66(20) 6.50(20) 1.34(18) 1.45(18) OH +13CH3F 2.26(43) 1.18(41) 8.99(41) 5.85(36) 6.51(31) 2.03(27) 5.37(25) OH + CD3F OD + CH3F OD + CD3F TST CVT/SCT TST CVT/SCT TST CVT/SCT 7.90(119) 5.50(87) 1.12(44) 2.12(44) 4.12(118) 1.62(86) 2.62(46) 1.83(45) 3.05(117) 6.00(86) 4.14(46) 3.06(45) 8.23(66) 4.51(54) 5.74(50) 1.19(37) 1.85(31) 1.04(27) 3.52(25) 3.92(43) 1.96(41) 1.47(40) 8.03(36) 7.67(31) 2.26(27) 5.78(25) 1.68(65) 7.56(54) 9.00(50) 1.54(37) 2.18(31) 1.17(27) 3.82(25) 1.26(43) 1.46(41) 1.43(40) 1.00(35) 9.16(31) 2.56(27) 6.34(25) 3.94(65) 1.38(53) 1.51(49) 2.04(37) 2.58(31) 1.30(27) 4.15(25) 2.19(43) 2.53(41) 2.43(40) 1.38(35) 1.08(30) 2.86(27) 6.85(25) 6.20(22) 7.97(22) 6.00(20) 6.90(20) 1.39(18) 1.51(18) OH +13CD3F 6.56(22) 6.29(20) 1.45(18) 8.40(22) 7.21(20) 1.57(18) 5.96(22) 7.62(22) 5.91(20) 6.77(20) 1.39(18) 1.50(18) OH + 14CH3F T(K) TST CVT/SCT TST CVT/SCT TST CVT/SCT 35 50 7.52(120) 1.14(87) 1.02(45) 2.09(45) 5.34(120) 8.90(88) 1.74(46) 3.97(46) 1.59(118) 1.25(86) 5.24(45) 1.03(44) 70 90 100 150 2.87(66) 2.09(54) 2.93(50) 8.11(38) 4.75(44) 2.85(42) 2.35(41) 3.28(36) 2.37(66) 1.79(54) 2.54(50) 7.32(38) 1.06(44) 7.50(43) 6.79(42) 2.08(36) 2.04(65) 1.18(53) 1.52(49) 3.34(37) 2.22(43) 1.29(41) 1.05(40) 1.30(35) 200 250 300 400 500 600 1.43(31) 8.63(28) 3.05(25) 5.37(22) 5.41(20) 1.28(18) a1.11(119) means 5.38(31) 1.80(27) 4.89(25) 6.79(22) 6.16(20) 1.38(18) 1.11 × 10119 1.32(31) 8.07(28) 2.88(25) 5.12(22) 5.20(20) 1.24(18) 4.61(31) 1.63(27) 4.52(25) 6.41(22) 5.88(20) 1.33(18) 5.32(31) 3.04(27) 1.04(24) 1.79(21) 1.79(19) 4.22(18) 1.94(30) 6.22(27) 1.65(24) 2.24(21) 2.02(19) 4.53(18) Table 10. Calculated KIEs by the TST and CVT/SCT methods of the OH + CH3F reaction in bulk water solvent. KIE(CD3) KIE(OD + CD3) KIE(OD) TST CVT/SCT TST CVT/SCT TST CVT/SCT TST CVT/SCT TST CVT/SCT KIE(13C) KIE(14C) KIE(13CD3) T(K) TST CVT/SCT 35 50 70 0.141 0.277 0.433 0.608 0.586 0.578 0.027 0.094 0.212 25.983 6.812 1.791 0.004 0.025 0.091 16.428 4.069 1.033 1.483 1.334 1.243 6.686 5.961 4.764 2.086 1.714 1.502 39.182 31.330 21.385 0.070 0.122 0.174 1.298 1.204 1.016 90 100 150 200 250 300 400 0.553 0.601 0.763 0.849 0.897 0.925 0.948 0.600 0.610 0.729 0.849 0.900 0.928 0.952 0.330 0.383 0.590 0.719 0.799 0.852 0.911 0.807 0.630 0.583 0.711 0.793 0.847 0.909 0.180 0.228 0.448 0.608 0.715 0.785 0.862 0.466 0.369 0.425 0.601 0.711 0.783 0.863 1.194 1.177 1.124 1.096 1.079 1.068 1.054 4.127 3.831 1.783 1.209 1.129 1.097 1.068 1.394 1.357 1.246 1.189 1.154 1.131 1.104 15.683 13.234 2.817 1.413 1.250 1.187 1.131 0.212 0.226 0.273 0.295 0.307 0.312 0.316 0.914 0.853 0.451 0.335 0.327 0.326 0.323 500 600 0.957 0.961 0.960 0.964 0.942 0.960 0.942 0.961 0.900 0.921 0.902 0.925 1.046 1.041 1.055 1.048 1.089 1.080 1.105 1.092 0.316 0.316 0.321 0.320 0 ΔV≠ Reactants Y + RX TS [Y∙∙∙R∙∙∙X] -10 -20 -30 30 Y∙∙∙RX X∙∙∙RY 20 ΔV≠ -40 -50 Products40 X + RY gas-phase 10 solution-phase 0 Reaction Coordinate -10 Scheme 1. Potential energy diagram of a typical gas-phase (black) and solution-phase (red) SN2 reactions. C3v C∞v C3v (a) (b) C3v (c) C3v Cs (d) Figure 1. Calculated structures by the MP2/aug-cc-pVTZ method of the gas-phase CN + CH3F reaction. Bond lengths are in Å (blue). Experimental values are in the parentheses. (a) Reactants (b) Products (c) Transition state (d) Ion-dipole complex. log k(T) (cm3 molecule1 s1) -20 CN + CH3F CN + CD3F CN + 13CH3F CN + 14CH3F -25 -30 -35 -40 -45 0 5 10 15 20 25 30 1000/T (K1) Figure 2. The Arrhenius plots of the calculated rate constants of the gas-phase CN + CH3F reaction. The broken and solid lines indicate results calculated at TST and CVT/SCT levels, respectively. 3.0 KIE(CD3) KIE(13C) KIE(14C) 2.5 KIE 2.0 1.5 1.0 0.5 0.0 0 5 10 15 1000/T 20 (K1) 25 30 Figure 3. Calculated temperature dependence of the KIEs of the gas-phase CN + CH3F reaction. The broken and solid lines indicate results calculated at TST and CVT/SCT levels, respectively. (a) (b) (c) (d) Figure 4. Calculated structures by the MP2/aug-cc-pVTZ method of the gas-phase OH(H2O) + CH3F reaction. Bond lengths are in Å (blue) and bond angles in degrees (red). (a) Reactants (b) Products (c) Transition state (d) Ion-dipole complex. Relative Energy (kcal/mol) 10 OH(H2O) + CH3F 0 -20 -∞ -15 -10 -5 Transition state 0 5 10 15 20 25 30 35 ∞ -10 Ion-dipole complex -20 F(H2O) + CH3OH -30 -40 Ion-dipole complex -50 s (Bohr) Figure 5. Calculated potential energy curves along the reaction path of the gas-phase OH(H2O) + CH3F reaction at the MP2/aug-cc-pVDZ level. log k(T) (cm3 molecule1 s1) -17 -19 -21 -23 -25 -27 -29 -15 log k(T) (cm3 molecule1 s1) OH(H2O) + CH3F OH(H2O) + CD3F OH(H2O) + 13CH3F OH(H2O) + 14CH3F -15 -17 -19 -21 -23 -25 -27 -29 -31 -31 -33 -33 -35 -35 0 5 10 15 20 1000/T (K1) 25 30 OH(H2O) + CH3F OD(H2O) + CH3F OH(D2O) + CH3F OD(D2O) + CH3F 0 5 10 15 20 1000/T (K1) 25 30 Figure 6. The Arrhenius plots of the calculated rate constants of the gas-phase OH(H2O) + CH3F reaction. The broken and solid lines indicate results calculated at TST and CVT/SCT levels, respectively. KIE(CD3) KIE(13C) KIE(14C) 9.0 8.0 0.9 0.8 7.0 0.7 KIE 6.0 KIE KIE(OD(H2O)) KIE(OH(D2O)) KIE(OD(D2O)) 1.0 5.0 0.6 0.5 4.0 0.4 3.0 0.3 2.0 0.2 1.0 0.1 0.0 0.0 0 10 20 1000/T (K1) 30 0 10 1000/T 20 (K1) 30 Figure 7. Calculated temperature dependence of the KIEs of the gas-phase OH(H2O) + CH3F reaction. The broken and solid lines indicate results calculated at TST and CVT/SCT levels, respectively. (a) (b) (c) Figure 8. Calculated structures by the MP2/aug-cc-pVTZ method of the OH + CH3F reaction in bulk water solvent. Bond lengths are in Å (blue) and bond angles in degrees (red). (a) Reactants (b) Products (c) Transition state. log k(T) (cm3 molecule1 s1) -25 -30 -35 -40 -45 -50 -20 log k(T) (cm3 molecule1 s1) OH + CH3F OH + CD3F OH + 13CH3F OH + 14CH3F -20 -25 -30 -35 -40 -45 -50 -55 -55 -60 -60 0 5 10 15 20 1000/T (K1) 25 30 OH + CH3F OD + CH3F OD + CD3F 0 5 10 15 20 1000/T (K1) 25 30 Figure 9. The Arrhenius plots of the calculated rate constants of the OH + CH3F reaction in bulk water solvent. The broken and solid lines indicate results calculated at TST and CVT/SCT levels, respectively. KIE(CD3) KIE(13CH3F) KIE(14CH3F) KIE(OD) KIE(OD + CD3) 4.0 3.5 KIE 3.0 2.5 2.0 1.5 1.0 0.5 0.0 0 10 1000/T 20 (K1) 30 Figure 10. Calculated temperature dependence of the KIEs of the OH + CH3F reaction in bulk water solvent. The broken and solid lines indicate results calculated at TST and CVT/SCT levels, respectively. CN + CH3F OH(H2O) + CH3F OH + CH3F (PCM, solvent=water) 20 VMEP (kcal/mol) 16.2 15 1.4, 8.2 1.1, 6.1 12.0 10 1.2, 8.1 5.1 1.2, 6.0 5 0.8, 2.5 0.8, 2.5 0 -5 -3 -1 -5 1 3 5 -10 -15 -20 s (bohr) Figure 11. The calculated dual-level energy profiles along the reaction path. The VMEP(s) of the half-width and the barrier heights are indicated in the figure. 3.5 3.0 2.5 2.0 t12C / tH t13C / tH t12C / tD t13C / tD 3.5 3.0 ratio ratio 4.0 t12C / tH t13C / tH t12C / tD t13C / tD 4.5 t12C / tH t13C / tH t12C / tD t13C / tD 4.0 3.5 3.0 2.5 ratio 4.0 2.5 2.0 2.0 1.5 1.5 1.5 1.0 1.0 0.5 0.5 0.0 0.0 0 50 100 150 200 250 300 T(K) (a) 1.0 0.5 0.0 0 50 100 150 200 250 300 T(K) (b) 0 50 100 150 200 250 300 T(K) (c) Figure 12. Temperature dependence of the calculated contributions from the tunneling effects of the rate constants for the 12C, and 13C of the (a) gas-phase CN + CH3F, (b) gas-phase OH(H2O) + CH3F, (c) OH + CHF in bulk water solvent.