word - Seattle Central College
advertisement

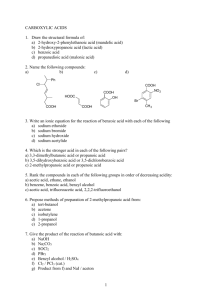
CHEM 122: Introduction to Organic Chemistry Chapter 11: Carboxylic Anhydrides, Esters, and Amides. 1. Draw a structural formula for each compound. a) b) c) d) e) f) Dimethyl carbonate p-Nitrobenzamide Ethyl 3-hydroxybutanoate Diethyl oxalate Ethyl trans-2-pentenoate Butanoic anhydride 2. Write the IUPAC name for each compound. O O O a) b) CH3(CH2)8COCH3 O H2N c) CH3(CH2)4CNHCH3 d) NH 2 O e) O f) CH3CHCH2COCH2CH3 3. What product forms when ethyl benzoate is treated with each of the following sets? a) H2O, NaOH, heat b) H2O, HCl, heat 4. What product forms when benzamide, C6H5CONH2, is treated with each of the following sets? a) H2O, NaOH, heat b) H2O, HCl, heat 5. Complete the equations for these reactions. O H 3 CO NH 2 + O a) O NH b) O O + O 6. The analgesic phenacetin is synthesized by treating 4-ethoxyaniline with acetic anhydride. Draw a structural formula for phenacetin. O NH 2 4-Ethoxyaniline 7. Phenobarbital is a long-acting sedative, hypnotic, and anticonvulsant. a) Name all functional groups in this compound. b) Draw structural formulas for the products from complete hydrolysis of all amide groups in aqueous NaOH. O H N O O N H Phenobarbital 8. Draw short sections of two parallel chains of nylon-66 (each chain running in the same direction) and show how it is possible to align them such that there is hydrogen bonding between the N—H groups of one chain and the C=O groups of the parallel chain. 9. Benzocaine, a topical anesthetic, is prepared by treating 4-aminobenzoic acid with ethanol in the presence of an acid catalyst, followed by neutralization. Draw a structural formula for benzocaine. 10. The analgesic acetaminophen is synthesized by treating 4-aminophenol with one equivalent of acetic anhydride. Write an equation for the formation of acetaminophen. (Hint: The –NH2 group is more reactive with acetic anhydride than the –OH group.) 11. Chapter 14 discusses a class of compounds called amino acids, so named because they contain both an amino group and a carboxyl group. Following is a structural formula for the amino acid alanine. O CH3CHCONH3+ Alanine What would you expect to be the major form of alanine present in aqueous solution a) at pH 2.0, b) at pH 5-6, and c) at pH 11.0? Explain. 12. Starting with ethylene as the only source of carbon atoms, show how you could synthesize ethyl acetate. 13. From what carboxylic acid and amine or ammonia can each amide be synthesized? O (CH 2 ) 4 CH 3 NH a) b) (CH3)2CHCN(CH3)2 c) H2NC(CH2)4CNH2 14. Following are structural formulas for two local anesthetics use in dentistry. Lidocaine was introduced in 1948 and is now the most widely used local anesthetic for infiltration and regional anesthesia. Its hydrochloride salt is marketed under the name Xylocaine. Mepivacaine is faster-acting and somewhat longer in duration than licodaine. Its hydrochloride salt is marketed under the name Carbocaine. NH N O a) b) c) d) NH N O Lidocaine Mepivacaine (Xylocaine) (Carbocaine) Name the functional groups in each anesthetic. Which of these anesthetics are chiral? Which nitrogen atom in each is the more basic? What similarities in structure do you find between these compounds?