Chemistry - Dry Cell & Lead-Acid
advertisement
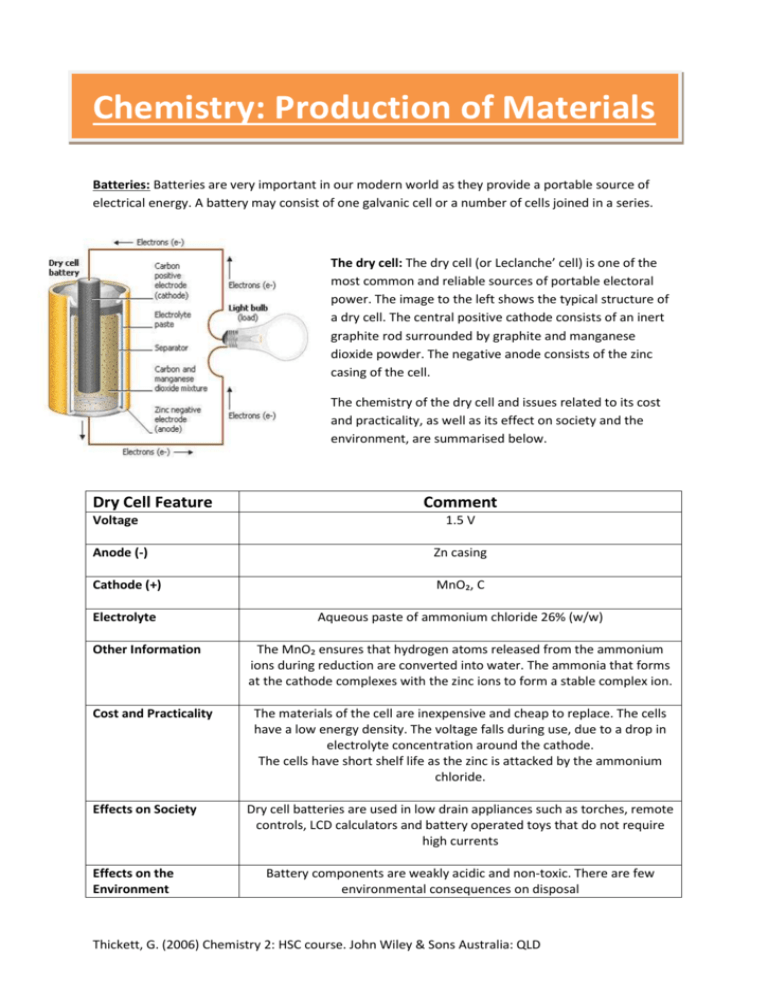
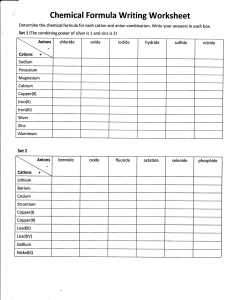
Chemistry: Production of Materials Batteries: Batteries are very important in our modern world as they provide a portable source of electrical energy. A battery may consist of one galvanic cell or a number of cells joined in a series. The dry cell: The dry cell (or Leclanche’ cell) is one of the most common and reliable sources of portable electoral power. The image to the left shows the typical structure of a dry cell. The central positive cathode consists of an inert graphite rod surrounded by graphite and manganese dioxide powder. The negative anode consists of the zinc casing of the cell. The chemistry of the dry cell and issues related to its cost and practicality, as well as its effect on society and the environment, are summarised below. Dry Cell Feature Voltage Comment 1.5 V Anode (-) Zn casing Cathode (+) MnO₂, C Electrolyte Aqueous paste of ammonium chloride 26% (w/w) Other Information Cost and Practicality Effects on Society Effects on the Environment The MnO₂ ensures that hydrogen atoms released from the ammonium ions during reduction are converted into water. The ammonia that forms at the cathode complexes with the zinc ions to form a stable complex ion. The materials of the cell are inexpensive and cheap to replace. The cells have a low energy density. The voltage falls during use, due to a drop in electrolyte concentration around the cathode. The cells have short shelf life as the zinc is attacked by the ammonium chloride. Dry cell batteries are used in low drain appliances such as torches, remote controls, LCD calculators and battery operated toys that do not require high currents Battery components are weakly acidic and non-toxic. There are few environmental consequences on disposal Thickett, G. (2006) Chemistry 2: HSC course. John Wiley & Sons Australia: QLD Follow this link for further basic yet useful information on dry cell batteries! http://www.wisegeek.com/what-is-a-dry-cell-battery.htm# http://www.youtube.com/watch?v=4l-2NcTbLBY Thickett, G. (2006) Chemistry 2: HSC course. John Wiley & Sons Australia: QLD