Olive Oil Tasting
advertisement

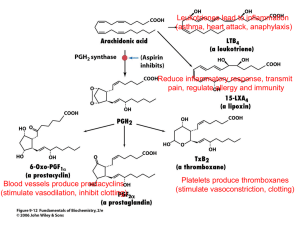
OLIVE OIL TASTING Please rate each of the following olive oils in terms of their "peppery" taste Olive Oil ibuprofen - 3 200mg tablets / 75 ml water Olave Organic (Chile) Les Moulins Mahjoub Organic (Tunisia) Carm (Portugal) Hojiblanca - Whole Foods (Spain) Badia a Cotibuono (Italy) Rating: 10 Nature. 2005 Sep 1;437(7055):45-6. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, Lee CH, Smith AB, Breslin PA. Monell Chemical Senses Center, Philadelphia, Pennsylvania 19104, USA. Abstract Newly pressed (ie more expensive) extra-virgin olive oil contains oleocanthal--a compound whose pungency induces a strong stinging sensation in the throat, not unlike that caused by solutions of the nonsteroidal anti-inflammatory drug ibuprofen. We show here that this similar perception seems to be an indicator of a shared pharmacological activity, with oleocanthal acting as a natural anti-inflammatory compound that has a potency and profile strikingly similar to that of ibuprofen. Although structurally dissimilar, both these molecules inhibit the same cyclooxygenase enzymes in the prostaglandinbiosynthesis pathway. see also: http://en.wikipedia.org/wiki/Oleocanthal http://www.3dchem.com/molecules.asp?ID=199 Good quality olive oil contains a natural chemical that acts in a similar way to a painkiller. The active ingredient - found in greater concentrations in fresher olives - is called oleocanthal and inhibits the activity of enzymes involved in inflammation in the same way as ibuprofen and other anti-inflammatory drugs. Inflammation has been linked to a wide range of conditions such as heart disease and cancer. To rule out the possibility that any other compound was involved, chemists at Monell and Penn created a synthetic form of oleocanthal identical in all respects to that found naturally in olive oil, and showed that it produced exactly the same throat irritation. The sensory similarities between oleocanthal and ibuprofen led scientists at Monell and the University of the Sciences to investigate potential common pharmacological properties. Studies revealed that, like ibuprofen, oleocanthal inhibits activity of COX-1 and COX-2 enzymes. Because inhibition of COX activity underlies the anti-inflammatory actions of ibuprofen and other non- steroidal anti-inflammatory drugs (NSAIDs), the new findings suggest oleocanthal is a natural antiinflammatory agent. Taking their lead from the cues provided by olive oil's throaty bite, the scientists systematically evaluated the sensory properties of an unnamed chemical compound thought to be responsible for the throat irritating property of premium olive oils. When results confirmed that the irritating intensity of a given extra-virgin olive oil was directly related to how much of the chemical it contained, the researchers named the compound oleocanthal (oleo=olive; canth=sting; al=aldehyde). http://www2.ups.edu/faculty/hanson/c251.05.projects/ibuprofen/ibuprofen.htm Ibuprofen is the active ingredient in a number of over the counter pain relievers, e.g. Advil, Motrin, and Nuprin. It is one of the top-ten drugs sold worldwide, and, although it has been shown that only the S enantiomer has the desired biological activity, it is currently sold as the racemate. http://www.chem.wisc.edu/areas/reich/syntheses/oleocanthal-smith-syn.htm (oleocanthal is an enantiomer; only the - form has he bitter taste) Pain Relief: *Natural pain killers (descending modulation from brain): enkephalins: produced by brain, spinal nerves -->inhibit release of substance P; endorphins & dynorphins: bind to opiate receptors in brain and spinal cord *Aspirin, ibuprofen (Advil®, Motrin®), naproxen (Aleve®) - block production of prostaglandins from arichidonic acid by inhibiting COX enzymes. (http://www.elmhurst.edu/~chm/vchembook/555prostagland.html) *Capsaicin (hot peppers)- “counterirritant” wh/ overstimulates type C fibers --> depletes substance P (http://pbs-saf.virage.com:80/cgi-bin/visearch?user=pbssaf&template=template.html&query=Life%27s+Little+Questions+1999&category=0&viKeyword=L ife%27s+Little+Questions+1999) *Local anesthetics - inhibit pain nerve cell pathway * Opiates - act in brain and spinal cord. Some examples: morphine, codeine , methadone, demerol, darvon. Opioids bind to receptors on interneurons in the pain pathways in the central nervous system. The natural ligands for these receptors are enkephalins. The enkephalins are released at synapses on neurons involved in transmitting pain signals back to the brain. Instead of synapsing with a dendrite or cell body, the enkephalin synapse occurs close to the terminal of a pain-signaling neuron. The enkephalins hyperpolarize the postsynaptic membrane thus inhibiting it from transmitting these pain signals. The activation of enkephalin synapses suppresses the release of the neurotransmitter (substance P) used by the sensory neurons involved in the perception of chronic and/or intense pain. * Acetaminophen - It selectively inhibits Cox-3 enzyme and provides pain relief without irritating the stomach; also acts in brain?? NonSteroidal Anti-Inflammatory Drugs (NSAIDs) The NSAIDs achieve their effects by blocking the activity of cyclooxygenase. In addition to reducing the fever and pain of inflammation, NSAIDs also inhibit clotting. They do this by interfering with the synthesis of thromboxane A2 in platelets. This is the reason that: *aspirin is given to patients undergoing angioplasty; *many men take a baby aspirin a day in the hope of avoiding heart attacks. But regular use of NSAIDs has a downside: a tendency to develop ulcers in the stomach and duodenum. COX-1 and COX-2 The body produces several different forms of cyclooxygenase (COX), including: *COX-1, which is involved in pain, clotting, and protecting the stomach; *COX-2, which is involved in the pain produced by inflammation. Most of the NSAIDs inhibit them both. However, some newer drugs, the so-called COX-2 inhibitors, such as *rofecoxib (Vioxx®) & celecoxib (Celebrex®) are much more active against COX-2 than COX-1. COX-2 inhibitors are effective against inflammation and seem to avoid damage to the GI tract. But, unfortunately, they increase the risk of heart attack because they do not block the synthesis of thromboxane A2 by platelets (which contain only COX-1). So people depending on NSAIDs for their heart protective effects must monitor any use of COX-2 inhibitors carefully. In fact, because of the increased risk of heart attacks (and also strokes), the manufacturer of Vioxx® removed it from the market on 30 September 2004. *Brain and Pain?? Individual perception (learned, cultural, gender?) placebo effect? Genetics? variations in COMT gene enzyme -->dopamine metabolism varies -->variation in endorphin levels Cyclooxygenase (COX) http://en.wikipedia.org/wiki/Cyclooxygenase