pro2450-sup-0001
advertisement
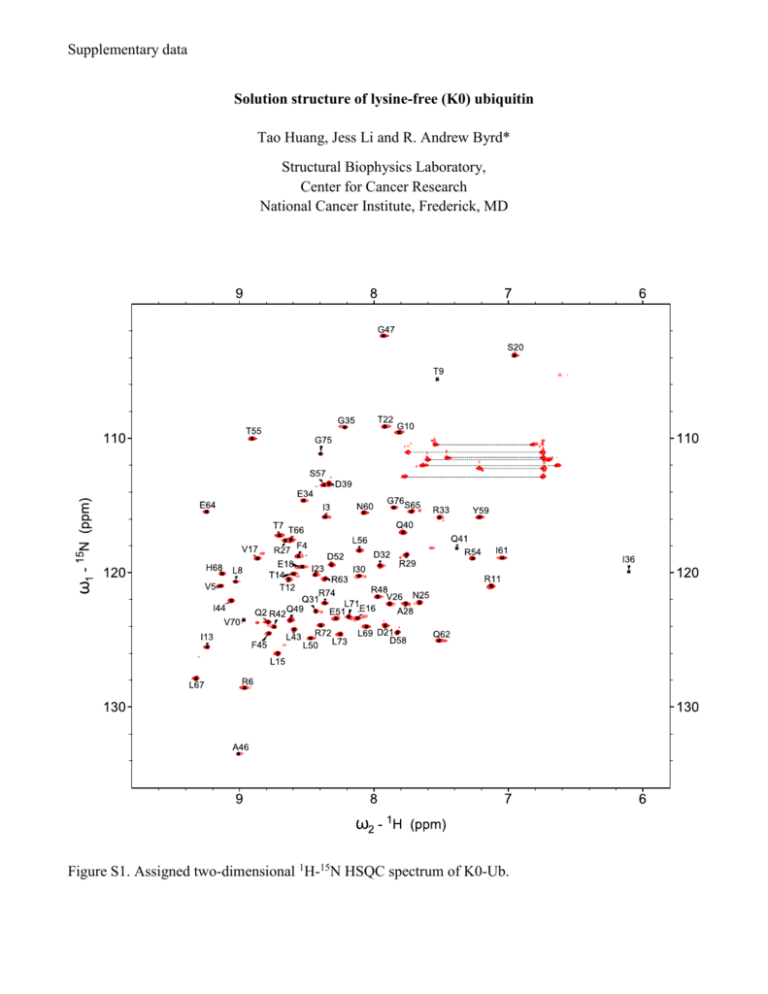
Supplementary data Solution structure of lysine-free (K0) ubiquitin Tao Huang, Jess Li and R. Andrew Byrd* Structural Biophysics Laboratory, Center for Cancer Research National Cancer Institute, Frederick, MD Figure S1. Assigned two-dimensional 1H-15N HSQC spectrum of K0-Ub. A talos_phi K0-Ub vs wt-Ub 100 Phi (K0-Ub) 50 0 -50 -100 -150 -150 -100 -50 0 50 100 150 200 Phi (wt-Ub) B talos_psi K0-Ub vs wt-Ub 200 Psi (K0-Ub) 150 100 50 0 -50 -50 0 50 100 Psi (wt-Ub) Figure S2. Plots of Phi (A) and Psi (B) angles of K0-Ub versus wt-Ub from TALOS calculation. One outlier on the psi plot was D52, which is involved in a salt bridged with K27 (Table S1). Figure S3. Thermal-induced unfolding curves of ubiquitin (Tm = 90.6 °C) and K0-Ub (Tm = 71.9 °C) measured by DSC. Table S1. Hydrogen bonding and salt-bridge interactions involving Lys NH3+ groups in wt-Ub (PDB: 1UBQ) Donor Acceptor Distance (Å) K11 Nζ E34 Oε 3.347 K27 Nζ Q41 O 3.245 K27 Nζ D52 Oδ 2.902 K29 Nζ E16 O 2.676 K33 Nζ T14 O 3.407 K48 Nζ A46 O 3.379 Concerning the issue of minor species in the spectra of K0-Ub: Anecdotally, there are differing amounts of minor conformation observed from different preparations of K0 ubiquitin, but the smallest minor populations always occur when the protein is purified from only the soluble fraction. When the protein is refolded, differing (and usually larger) amounts of minor species are observed. Since ubiquitin is so evolutionarily conserved and stable, consistent with its role as a posttranslational modifier, it is likely that the form purified from the soluble fraction following expression represents the low energy form. Consequently, we feel that the structure reported represents this form and has been shown to be equivalent to wild-type ubiquitin. When a significant number of alterations affecting H-bonds and salt-bridges occur throughout the protein, it is possible to trap minor populations with varying orientations of some of these components. The data suggests that there is not a single population for the minor species, and it is most likely that the ensemble of molecules represents cases where the major conformation exists for most of the residues and a small number of residues in any given molecule exhibit a different conformation or perturbation of an H-bond or salt-bridge. In this situation, there can be different percentages of minor conformations at different residues without presupposing that there is a single major species and a single minor species. Thus, the most stable conformation is represented by the major peaks in the spectrum, and the minor peaks represent low populations of alternate conformations, which may exist in isolated segments within the ensemble, rather than a single alternate conformation. This view is supported both by the nature of the perturbations and the fact that there is a range of populations observed across the residues in Ubiquitin. The population of “assignable” minor species ranges from 4% to 26%. We have prepared a plot of the CSP for the minor species relative to the wild-type Ubiquitin. This shows that there are perturbations distributed through the structure. The scale of the perturbations is smaller than for the major species, but not disparately so. This plot is somewhat misleading, as it does not express the population of the species/peaks. We can assign peaks in triple resonance spectra for 27 residues, with populations ranging from 4% to 26% (indicated in the CSP plot by red bars, referenced to the right vertical axis). A very interesting aspect of these assignments is that it is impossible to unambiguously assign the minor peaks to a single molecular species. First, the population variation suggests that there is not a single molecular species representing the minor conformation. Second, the triple resonance spectra exhibit degeneracy for the Ca, Cb, and C’ shifts, hence, it is impossible to trace a connectivity through only the minor peaks. Instead, it is more likely that the minor conformations are distributed with perhaps one or a few alternate forms in any single molecular species that is, otherwise, predominantly the major form. The rate of exchange is clearly slow on the NMR timescale, and, based on the chemical shift separation (as small as 0.015 ppm [15N]), the rate is slower than 1.05 sec-1. Careful examination of the minor resonances, which are populated at more than 10%, suggests that the chemical shifts are most likely due to local perturbations in a side-chain to side-chain hydrogen bond formed by a Lys sidechain that is mutated to Arg in K0-Ub or a salt-bridge. Replacement of the Lys ξ-amino group with the Arg guanadino moiety could lead to either loss of the hydrogen bond or compensation to pack in the bulkier sidechain and the possibility of two inequivalent rotamers of the Arg sidechain enabling the formation of the hydrogen bonds via either the η1 or η2 amino groups. Indeed, some of the perturbations are correlated with an approximately equal population, such as those of I13 and L15 with R33 and R29 (these are R in the K0-Ub). Based on this analysis of the data from the minor species, it is clear that the principal tenet of our manuscript is maintained, namely that the three-dimensional structure of K0-Ub is equivalent to wtubiquitin. While intriguing and potentially the subject of subsequent detailed investigation, which could contribute to the understanding of protein folding and stability, we respectfully submit that this goes beyond the nature of this short communication of a valuable new protein structure. The stability of the K0-Ub fold, as well as the equivalence for the fold of the minor conformers (based on the Ca, Cb, C’ shifts and the respective torsion angles from TALOS), suggests that K0-Ub is indeed a viable molecular replacement for wt-ubiquitin in biochemical and cellular studies, where the ability to control ubiquitination and chain extension is desired.