Microsoft Word - Chem.2nd.Six.Wks.14.15
advertisement

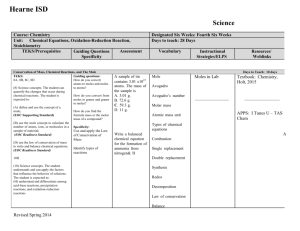
Hearne ISD Science Course: General Chemistry Units: Atoms, The Mole, Arrangement of Electrons (Electromagnetic Spectrum and Electron Configuration) TEKS/Prerequisites Guiding Questions Assessment / Specificity The Mole TEKS: 8A, 8B, 8C (8) Science concepts. The student can quantify the changes that occur during chemical reactions. The student is expected to: (A) define and use the concept of a mole; (EOC Supporting Standard) (B) use the mole concept to calculate the number of atoms, ions, or molecules in a sample of material; (EOC Readiness Standard) Designated Six Weeks: Second Six Weeks Days to teach: 28 Days Vocabulary Instructional Strategies/ELPS Days to Teach: 15 Days Guiding questions: How do you convert atoms to moles and moles to atoms? How do you convert from moles to grams and grams to moles? How do you find the formula mass or the molar mass of a compound? A sample of tin contains 3.01 x1023 atoms. The mass of the sample is A. 3.01 g. B. 72.6 g. C. 59.3 g. D. 11 g. Mole Avogadro Avogadro’s number Molar mass Atomic mass unit Molecule Specificity: Use isotopic composition to calculate average atomic mass of an element. Exemplar Lesson Average Atomic Mass TEKS 8 A,B Mole Lab Guiding questions: (6) Science concepts. The student knows and understands the historical development of atomic theory. The student is expected to: How are wavelength, frequency, and speed of light related? (C=wavelength x Frequency) Revised Spring 2014 Holt One Stop Planner APPS: I Tunes U – TAS Chem A Days: 7 TEKS: 6B, 6C (C) Calculate the wavelength, frequency, and energy of light using Text: Modern Chemistry, Holt, 2015. Exemplar Lesson Percent Composition Lab TEKS 8B Electromagnetic Radiation (B) Understand the electromagnetic spectrum and the mathematical relationships between energy, frequency, and wavelength of light; (EOC Supporting Standard) Resources/ Weblinks What is a photon, and how does it relate to the equation E = hv? How does the If red light has a frequency of 725 nm, calculate the energy of a single photon of the red light. Wavelength Describe a quanta as it relates to electrons and atoms. Spectrum Frequency Quantum Photon Energy Speed of light Atomic theory Spectral Tubes Lab Exemplar Lesson Flame Test Lab ELPS: http://ritter.tea.state.t x.us/rules/tac/chapter 074/ch074a.html 2H – comprehension strategies Electromagnetic Spectru http://science.hq.nasa.gov kids/imagers/ems/index.ht ml m Hearne ISD Science Course: General Chemistry Units: Atoms, The Mole, Arrangement of Electrons (Electromagnetic Spectrum and Electron Configuration) TEKS/Prerequisites Guiding Questions Assessment / Specificity Planck's constant and the speed of light; (EOC Supporting Standard) College and Career Readiness Standard photoelectric effect correlate to the wave particle duality of light? Designated Six Weeks: Second Six Weeks Days to teach: 28 Days Vocabulary Atom Proton Instructional Strategies/ELPS Resources/ Weblinks 4K – labs 5G – daily oral language Specificity: Neutron B. Atomic Structure 1.Summarize the development of atomic theory. Understand the EM spectrum and apply to the speed of light and energy equations. Electron Understand that models of the atom are used to help understand the properties of elements and compounds. Electron Configuration TEKS: 6E, 7D (6) Science concepts. The student knows and understands the historical development of atomic theory. The student is expected to: (E) Express the arrangement of electrons in atoms through electron configurations and Lewis valence electron dot structures. (EOC Readiness Standard) (D) describe the nature of metallic bonding and apply the theory to explain metallic properties such as thermal and electrical conductivity, malleability, and ductility; (EOC Supporting Standard) Revised Spring 2014 Days to Teach: 6 Guiding questions: What are the major rules for writing electron configurations? Describe the arrangement of electrons in metallic bonding. Specificity: Write electron configurations and abbreviated (noble gas) configurations. Highlight exceptions. Describe s, p, d, and f blocks Write the complete electron configuration for lead. Orbital Electron Configuration Ground state ELPS: http://ritter.tea.state.t x.us/rules/tac/chapter 074/ch074a.html 1E – expert novice 3D – question answer 3E – think, pair, share Text: Modern Chemistry, Holt, 2015. APPS: I Tunes U – TAS A Chem Electron Configuration http://www.chemcollectiv .org/applets/pertable.php