File - Caitlin Keller`s Portfolio
advertisement
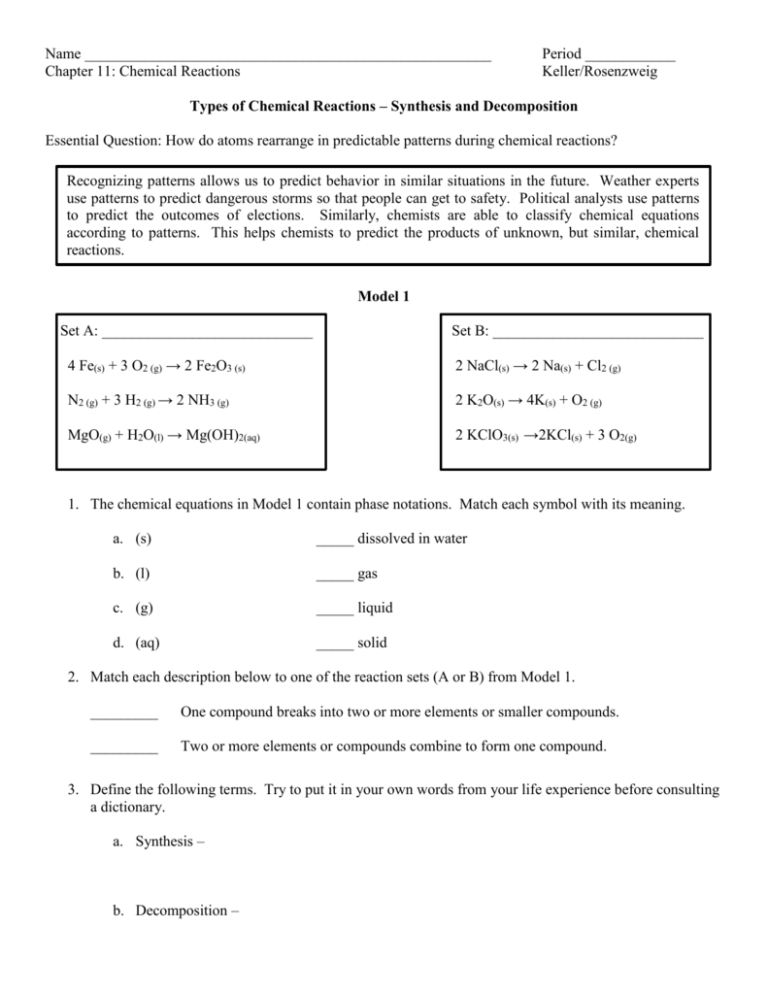
Name ______________________________________________________ Chapter 11: Chemical Reactions Period ____________ Keller/Rosenzweig Types of Chemical Reactions – Synthesis and Decomposition Essential Question: How do atoms rearrange in predictable patterns during chemical reactions? Recognizing patterns allows us to predict behavior in similar situations in the future. Weather experts use patterns to predict dangerous storms so that people can get to safety. Political analysts use patterns to predict the outcomes of elections. Similarly, chemists are able to classify chemical equations according to patterns. This helps chemists to predict the products of unknown, but similar, chemical reactions. Model 1 Set A: ____________________________ Set B: ____________________________ 4 Fe(s) + 3 O2 (g) → 2 Fe2O3 (s) 2 NaCl(s) → 2 Na(s) + Cl2 (g) N2 (g) + 3 H2 (g) → 2 NH3 (g) 2 K2O(s) → 4K(s) + O2 (g) MgO(g) + H2O(l) → Mg(OH)2(aq) 2 KClO3(s) →2KCl(s) + 3 O2(g) 1. The chemical equations in Model 1 contain phase notations. Match each symbol with its meaning. a. (s) _____ dissolved in water b. (l) _____ gas c. (g) _____ liquid d. (aq) _____ solid 2. Match each description below to one of the reaction sets (A or B) from Model 1. _________ One compound breaks into two or more elements or smaller compounds. _________ Two or more elements or compounds combine to form one compound. 3. Define the following terms. Try to put it in your own words from your life experience before consulting a dictionary. a. Synthesis – b. Decomposition – 4. Based on your answers to questions #2 and #3, label Set A and Set B as either “Synthesis Reaction” or “Decomposition Reaction” in Model 1 above. 5. Can two elements be used as reactants for a synthesis reaction? If yes, give an example from Model 1. 6. Can two compounds be used as reactants for a synthesis reaction? If yes, give an example from Model 1. 7. What types of substances (elements, compounds, or both) are seen as products in decomposition reactions? Use two examples from Model 1. 8. Choose one example from the set of synthesis reactions in Model 1. a. Write the chemical reaction in reverse so that the reactants become the products and vice versa. b. What type of reaction is shown from what you wrote in part a? 9. Identify each of the reactions below as synthesis (S) or decomposition (D). a. ______ K2O(s) + H2O(l) → 2 KOH(aq) b. ______ 2 Al2O3 (s) → 4 Al(s) + 3 O2 (g) c. ______ 2 NaF(s) → 2 Na(s) + F2 (g) d. ______ 2 O2(g) + N2 (g) → N2O4 (g) 10. A student writes the following incorrect chemical equation for the synthesis of magnesium oxide: Mg + O2 → MgO2 a. What is the correct formula for elemental (when oxygen is found alone) oxygen? b. What is the correct formula for magnesium oxide (as an ionic compound)? c. Describe the error made by the student. d. Write the correct balanced equation for the synthesis of magnesium oxide. 11. Consider the following chemical reaction written as a word equation: diphosphorus pentoxide + water → phosphoric acid a. Identify the type of chemical reaction (synthesis or decomposition). b. Write the chemical formulas under the names of the substances in the word equation above. c. Balance the equation by using coefficients. Chemists use their knowledge of synthesis and decomposition reactions to predict what will happen in other, similar situations. When predicting the products for a reaction, it is important to remember that atoms will only combine in ways that make them stable. This means that it is important to pay attention to the charges on ions (when writing ionic compounds) and the natural state of elements (diatomic vs. singular) It is only after predicting the products and writing correct formulas that a chemist would balance the equation by using coefficients. When predicting products for questions 12-15: 1. Write the reactants as chemical formulas. 2. Determine the type of reaction – synthesis or decomposition. 3. If synthesis, there will only be one product. If the reactants contained a metal and a nonmetal, the product will be an ionic compound (most common). Criss-cross the charges to get the formula. If the reactants contained two nonmetals, the product will be a covalent compound. Use prefixes. 4. If decomposition, there will be multiple products. For the ones you are expected to know, the products will be elements. Write the chemical formulas, making sure to correctly write the diatomic elements (Br2, I2, N2, Cl2, H2, O2, F2). 5. Balance the equation using coefficients. 12. aluminum + nitrogen → ? 13. dinitrogen monoxide → ? 14. sodium + oxygen → ? 15. barium chloride → ?