Name: Mandi Lawrence Name of Demonstration: Solar Purifier
advertisement

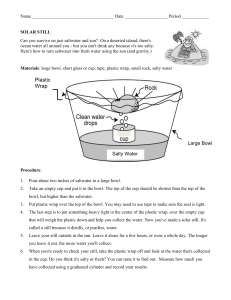
Name: Mandi Lawrence Name of Demonstration: Solar Purifier Materials: Water Salt Large Bowl Short Glass (Something that can easily fit inside the larger bowl) Plastic Wrap Tape (optional) Small Rock Procedure: 1) Mix salt with two cups of water until all of the salt is dissolved, then pour your mixture into the large bowl. 2) Place your short glass (opening up) in the middle of the larger bowl. The glass should not be as high as the rim of the bowl but should be tall enough that salt water does not get into it. 3) Stretch the plastic wrap over the large bowl. Make sure you have a tight seal around the rim of the bowl- you may need to use tape. 4) Place your small rock on top of the plastic wrap directly above the short glass. 5) Set the bowl outside in the sun for at least a few hours. When you return, there should be water in the glass. You can taste it to determine whether the water is salty or not (it shouldn’t be). The longer you leave the bowl out in the sun, the more water will be transferred from the bowl to the short glass. Potential Observations: This demonstration should result in the small glass having fresh water inside it while the salt is left behind in the bottom of the bowl. The sun warms the water until it evaporates and transforms into a gas. The gas then condenses on the plastic and rolls towards the weight or rock, eventually dropping off into the glass. This is similar to how water vapor condenses into clouds and then is returned to Earth through rain. Conclusion: This demonstration can help students to understand the processes of evaporation and condensation. It also demonstrates how saltwater here on Earth is transformed into clean, drinkable rain water. This is also handy to know how to do, in case you’re ever desperately in need of fresh water and don’t have access to it. Similar Experiments: This experiment can also be done using dirt or sand rather than salt. To vary the experiment a little, you could set a bowl of salty water outside for a few days without any plastic covering it. After all of the water evaporates, the only thing left will be salt in the bottom of the bowl. This demonstrates the evaporation process while leaving out the condensation process. Another experiment can be done by heating a pan of hot water on top of a burner, with two trash cans on either side of the burner. You place a cookie sheet so that it completely covers the pan of water and place ice cubes on the cookie sheet. Since this creates a cool surface, the steam rising from the pan will condense onto the cookie sheet and eventually fall back into the pan.