Chapter 01
advertisement

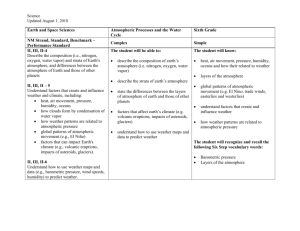
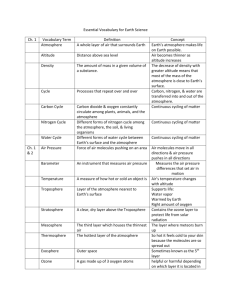
1 Instructor's Manual and Test Bank to accompany Meteorology Today, 10th Edition Jonathan D. W. Kahl University of Wisconsin-Milwaukee Chapter 1 Earth and Its Atmosphere Summary With many illustrations and photographs, this introductory chapter presents a broad overview of the physical structure of the atmosphere and its weather. The chapter begins with a discussion of the present composition of the earth's atmosphere. A focus section, “A Breath of Fresh Air”, examines the number of molecules in a single breath and in the entire atmosphere. The important and varied roles played by water vapor, which is a source of precipitation and latent heat energy as well as being the most important greenhouse gas, are given particular attention. Current concern over increasing concentrations of another constituent, carbon dioxide, and its possible effect on global climate are also examined. The student will see that the observed increase in CO2 is a result of an imbalance between processes of release and removal. The principle atmospheric pollutants, including ozone, are listed but are covered in greater detail in Chapter 18. The concepts of air density and air pressure are introduced and their variation with altitude is examined. A vertical profile of temperature shows that the atmosphere can be divided into several layers with distinct properties. Additional focus sections describe “The Atmospheres of Other Planets” and “The Radiosonde”. Finally, the student is introduced to the elements that constitute weather and will see how weather conditions might be depicted on a surface weather map and in a photograph from a geostationary satellite. The chapter includes discussions of the history of and careers in meteorology, and ends with a description of the many ways that weather and climate can affect our lives and interests. The final section includes a focus section entitled “What is a Meteorologist”. 2 Teaching Suggestions 1. also. Some of the atmospheric pressure demonstrations described in Chapter 8 could be performed here 2. Fill a wine glass completely with water and cover it with a piece of plastic (such as the lid from a tub of margarine), being careful to remove any air. Invert the glass. The water remains in the glass because the upward force on the cover due to the pressure of the air is much stronger than the downward gravitational force on the water. The demonstration can be made much more convincing if a 4000 mL Erlenmeyer flask is used instead of the wine glass. When full of water, the flask weighs approximately 10 pounds. 3. Place a candle in the center of a dish and partly fill the dish with water. Light the candle and then cover it with a large jar or beaker. The flame will consume the oxygen inside the jar and reduce the pressure. Water will slowly flow into the jar to re-establish pressure balance. The change in volume will be close to 20%, the volume originally occupied by the oxygen in the air. This demonstration can be used to illustrate the concept of partial pressure, which is later used in the chapter on humidity. The students should also be asked what they think the products of the combustion might be and why these gases do not replace the oxygen and maintain the original pressure in jar. One of the combustion products is water vapor, which condenses as the air in the jar cools. Another combustion product is carbon dioxide, which presumably goes into solution. 4. Students often confuse water vapor with liquid water. Students should understand that water vapor is an invisible gas. Haze, fog, clouds, and the steam from a boiling pot all become visible when water vapor condenses and forms small drops of liquid water. This can be easily demonstrated using a tea kettle, or by showing a video of water boiling in a tea kettle. 5. The introductory explanations of the air motions associated with high and low pressure centers and fronts make this a good place to begin to show and discuss satellite images, loops, and surface weather maps. Download a few satellite loops and discuss the air motions. 6. Challenge students to speculate on how we know the chemical composition of the earth’s early atmosphere. 7. Invoke the concept of layering while describing the composition of atmospheric gases. Nitrogen comprises 78% of the atmosphere, so if the gases each occupied distinct layers with Nitrogen on the bottom, the Nitrogen layer would extend from the floor up to about ¾ the distance to the ceiling. 8. Compare the thickness of the atmosphere to that of the skin on an apple. If the earth & atmosphere combined were shrunk down to the size of an apple, the atmosphere would be about as thick as the skin on the apple. 9. Compare the weather changes occurring over vertical and horizontal distances. If you moved 10 km to the north, south, east or west, what weather changes might you experience? Compare this with the weather changes you’d experience if you moved up 10 km. 10. Use the University of Wyoming’s radiosonde data website to examine current sounding data for anywhere in the world: http://weather.uwyo.edu/upperair/sounding.html. 3 Student Projects 1. Have the students mark the positions of fronts and pressure systems for each day on an outline map of the United States. (This information can be obtained from the daily newspaper or from the web.) Have students do this for a week at a time, noting the general movement of these systems. 2. Students could attempt to repeat some of the experiments in the book Hands-On Meteorology. 3. "A General Chemistry Experiment for the Determination of the Oxygen Content of Air" by James P. Birk, Larry McGrath, and S. Kay Gunter (J. Chem. Educ., 58, 804-805, 1981) describes a simple experiment that can be used to determine atmospheric oxygen concentrations (see also: "Percent Oxygen in Air," George F. Martins, J. Chem. Educ., 64, 809-810, 1987). 4. Have students compose a one-week journal or blog, including daily newspaper or web weather maps and weather forecasts. Ask them to write a commentary for each day as to the coincidence of actual and predicted weather. 5. Have students keep a daily record of weather conditions that they actually observe. Th en, periodically, the instructor can supply mean daily data such as high and low temperatures, pressure, dew point, wind speed, cloud cover, and precipitation amounts. The students should plot this data and annotate the graph with their observations. Students can use their graphs to experimentally test concepts developed in class. After studying Chapter 1, for example, students might try to determine whether periods of stormy weather really are associated with lower-than-average surface pressure. Answers to Questions for Review 1. Radiant energy from the sun. 2. Nitrogen, oxygen, argon, and water vapor. 3. Water vapor. 4. It forms precipitation, releases latent heat, and is a greenhouse gas. 5. Enters the atmosphere through vegetation decay, volcanic eruptions, exhalations of animal life, burning of fossil fuels, and deforestation. Removal is by photosynthesis and deposition to oceans. Increasing CO2 due to burning of fossil fuels and deforestation. 6. Water vapor and carbon dioxide. They are greenhouse gases because they absorb infrared radiation. 7. Ozone and oxygen filter out damaging ultraviolet radiation from the sun; greenhouse gases keep the planet warm; it provides water to drink and oxygen to breathe. 8. Soil dust, salt from ocean waves, forest fire smoke, volcanic ash particles, pollutants. 4 9. The earth’s first atmosphere (some 4.6 billion years ago) was most likely hydrogen and helium— the two most abundant gases found in the universe—as well as hydrogen compounds, such as methane and ammonia. A second, more dense atmosphere gradually enveloped the earth as gases from molten rock within its hot interior escaped through volcanoes and steam vents. We assume that volcanoes spewed out the same gases then as they do today: mostly water vapor (about 80 percent), carbon dioxide (about 10 percent), and up to a few percent nitrogen. As millions of years passed, the constant outpouring of gases from the hot interior (outgassing) provided a rich supply of water vapor, which formed into clouds. Rain fell upon the earth for many thousands of years. Large amounts of CO2 were dissolved in the oceans. The atmosphere gradually became rich in nitrogen (N2). Oxygen (O2), the second most abundant gas in today’s atmosphere, probably began an extremely slow increase in concentration as energetic rays from the sun split water vapor (H2O) into hydrogen and oxygen during a process called photodissociation. The hydrogen, being lighter, probably rose and escaped into space, while the oxygen remained in the atmosphere. After plants evolved, the atmospheric oxygen content increased more rapidly, probably reaching its present composition about several hundred million years ago. 10. (a) If more molecules are packed into an air column, the column becomes more dense, the air weighs more, and the surface pressure goes up. On the other hand, when fewer molecules are in the air column, the air weighs less, and the surface pressure goes down. (b) Because as altitude increases, there is always less air above you (because more of it is below you). 11. 1013.25 mb = 1013.25 hPa = 29.92 in. Hg. 12. About 6.5°C for every 1000 m or about 3.6°F for every 1000 ft rise in elevation. 13. On average, temperature decreases from the surface to the tropopause (around 10 km, then increases to the stratopause (around 50 km), then decreases to the mesopause (around 90 km), then increases through the thermosphere. 14. Troposphere, stratosphere, mesosphere, thermosphere. 15. Troposphere. 16. (a) Mesosphere. (b) Thermosphere. (c) Stratosphere. 17. Antarctica. 18. The lower part (called the D region) reflects standard AM radio waves back to earth, but at the same time it seriously weakens them through absorption. At night, though, the D region gradually disappears and AM radio waves are able to penetrate higher into the ionosphere (into the E and F regions) where the waves are reflected back to earth. Because there is, at night, little absorption of radio waves in the higher reaches of the ionosphere, such waves bounce repeatedly from the ionosphere to the earth’s surface and back to the ionosphere again. In this way, standard AM radio waves are able to travel for many hundreds of kilometers at night. 19. Because the pressure is so low in the upper stratosphere, 21 percent of a very small amount of air does not provide enough oxygen to breathe. 5 20. Meteorology is the study of the atmosphere and its phenomena. The term itself goes back to the Greek philosopher Aristotle who, about 340 B.C., wrote a book on natural philosophy entitled Meteorologica. 21. From the south. 22. High and low pressure systems, fronts, wind speed and direction, cloud cover, temperatures. 23. Low pressure systems: counterclockwise. High pressure systems: clockwise. 24. A sharp change in temperature, humidity, and wind direction. 25. Middle-latitude cyclonic storm, hurricane, thunderstorm, tornado. 26. From west to east. 27. Climate describes weather conditions averaged over a region or over a time period. 28. Answer not provided. Answers to Questions for Thought 1. Weather: (b), (d), (g). Climate: (a), (c), (e), (f), and (h). 2. (a) 0.5 ATM and 0.1 ATM are equal to about 500 mb and 100 mb, respectively. From the figure, 500 mb is located at an altitude of about 5.5 km (3.5 miles); 100 mb is found at an altitude of about 16 km (10 miles). (b) The surface pressure on Mars, 0.007 ATM, is about 7 mb. A pressure of 7 mb would be found near 35 km (22 miles) altitude in the earth's atmosphere. 3. Your stomach would expand, because the pressure outside your body would be several orders of magnitude less than the pressure inside your body. Answers to Critical Thinking Questions Figure 1.13. The sound travels in waves through the ionosphere. In some locations the waves pass closer to the earth’s surface than in other areas. Figure 1.14. Since weather tends to move from west to east, people in Centerville generally experience the weather that occurred to the west of them. The hail is about 80 miles west of Centerville and should reach Centerville in 2 hours. 6 Multiple Choice Exam Questions 1. The primary source of energy for the earth's atmosphere is a. energy from within the earth. b. the sun. c. erupting volcanoes. d. lightning discharges associated with thunderstorms. e. latent heat released during the formation of hurricanes. ANSWER: B 2. The most abundant gases in the earth's atmosphere by volume are a. carbon dioxide and nitrogen. b. oxygen and water vapor. c. nitrogen and oxygen. d. oxygen and helium. e. oxygen and ozone. ANSWER: C 3. Water vapor is a. a gas. b. a cloud droplet. c. a rain drop. d. a snowflake. ANSWER: A 4. In a volume of air near the earth's surface, ____ occupies 78 percent and ____ nearly 21 percent. a. nitrogen, oxygen b. hydrogen, oxygen c. oxygen, hydrogen d. nitrogen, water vapor e. hydrogen, helium ANSWER: A 5. The earth's rotation has ____ to do with the behavior of atmospheric storms. a. nothing b. little c. much ANSWER: C 6. Which of the following is considered a variable gas in the earth's atmosphere? a. water vapor b. nitrogen c. oxygen 7 d. argon e. helium ANSWER: A 7. The gas that shows the most variation from place to place and from time to time in the lower atmosphere is a. ozone (O3). b. carbon dioxide (CO2). c. water vapor (H2O). d. methane (CH4). e. argon (Ar). ANSWER: C 8. Water vapor a. is invisible. b. colors the sky blue. c. makes clouds white. d. is very small drops of liquid water. ANSWER: A 9. Typically, water vapor occupies about what percentage of the air's volume near the earth's surface? a. about 78 percent b. about 21 percent c. close to 10 percent d. less than 4 percent ANSWER: D 10. The only substance near the earth's surface that is found naturally in the atmosphere as a solid, liquid, and a gas is a. carbon dioxide. b. water. c. molecular oxygen. d. ozone. e. carbon. ANSWER: B 11. The most abundant greenhouse gas in the earth's atmosphere is a. carbon dioxide (CO2). b. nitrous oxide (N2O). c. water vapor (H2O). d. methane (CH4). e. chlorofluorocarbons (CFCs). 8 ANSWER: C 12. Since the turn of this century, CO2 in the atmosphere has a. been increasing in concentration. b. been decreasing in concentration. c. remained at about the same concentration from year to year. d. disappeared entirely. ANSWER: A 13. Which of the following processes acts to remove carbon dioxide from the atmosphere? a. lightning b. deforestation c. photosynthesis d. burning fossil fuels ANSWER: C 14. The primary source of oxygen for the earth's atmosphere during the past half billion years or so appears to be a. volcanic eruptions. b. photosynthesis. c. photodissociation. d. exhalations of animal life. e. transpiration. ANSWER: B 15. ____ holds a planet's atmosphere close to its surface. a. Radiation b. Gravity c. Cloud cover d. Moisture e. Pressure ANSWER: B 16. Much of Tibet lies at altitudes over 18,000 feet where the pressure is about 500 mb. At such altitudes, the Tibetans are above roughly a. 10 percent of the air molecules in the atmosphere. b. 25 percent of the air molecules in the atmosphere. c. 50 percent of the air molecules in the atmosphere. d. 75 percent of the air molecules in the atmosphere. e. 90 percent of the air molecules in the atmosphere. ANSWER: C 17. The unit of pressure most commonly found on a surface weather map is 9 a. b. c. d. inches of mercury (Hg). millibars or hectopascals. pounds per square inch. millimeters of mercury (Hg). ANSWER: B 18. Which of the following weather elements ALWAYS decreases as we climb upward in the atmosphere? a. wind b. temperature c. pressure d. moisture e. all of the above ANSWER: C 19. The gas responsible for the greenhouse effect on Venus is a. carbon dioxide (CO2). b. oxygen (O2). c. ozone (O3). d. nitrogen (N2). e. water vapor (H2O). ANSWER: A 20. The planet with a strong greenhouse effect, whose surface temperature averages 480°C (900°F) is a. Earth. b. Venus. c. Mars. d. Pluto. ANSWER: B 21. The earth's atmosphere is divided into layers based on the vertical profile of a. air pressure. b. air temperature. c. air density. d. wind speed. ANSWER: B 22. Carbon dioxide is a naturally-occurring component of the atmosphere. a. true b. false ANSWER: A 10 23. Almost all of the earth's weather occurs in the a. exosphere. b. stratosphere. c. mesosphere. d. thermosphere. e. troposphere. ANSWER: E 24. The most abundant gas in the stratosphere is a. oxygen (O2). b. nitrogen (N2). c. carbon dioxide (CO2). d. ozone (O3). e. chlorofluorocarbons (CFCs). ANSWER: B 25. The atmospheric layer in which we live is called the a. troposphere. b. stratosphere. c. thermosphere. d. ionosphere. e. exosphere. ANSWER: A 26. The temperature of the tropopause a. is close to the temperature at the earth's surface. b. is much colder than the temperature at the earth's surface. c. has never been measured. d. is much warmer than the temperature at the earth's surface. e. is nearly the same as the sun's temperature. ANSWER: B 27. In a temperature inversion a. air temperature increases with increasing height. b. air temperature decreases with increasing height. c. air temperature remains constant with increasing height. d. it is warmer at night than during the day. ANSWER: A 28. The rate at which temperature decreases with increasing altitude is known as the a. temperature slope. b. lapse rate. c. sounding. 11 d. thermocline. ANSWER: B 29. Most of the ionosphere is found in what atmospheric layer? a. troposphere b. stratosphere c. mesosphere d. thermosphere ANSWER: D 30. The gas that absorbs most of the harmful ultraviolet radiation in the stratosphere is a. water vapor. b. nitrous oxide. c. carbon dioxide. d. ozone. e. chlorofluorocarbons. ANSWER: D 31. Which latitude belt best describes the middle latitudes? a. 20° to 80° b. 10° to 35° c. 20° to 35° d. 40° to 70° e. 30° to 50° ANSWER: E 32. The word "weather" is defined as a. the average of the weather elements. b. the climate of a region. c. the condition of the atmosphere at a particular time and place. d. any type of falling precipitation. ANSWER: C 33. The wind direction is a. the direction from which the wind is blowing. b. the direction to which the wind is blowing. c. always directly from high toward low pressure. d. always directly from low toward high pressure. ANSWER: A 34. Meteorology is the study of a. landforms. 12 b. c. d. e. the oceans. the atmosphere. outer space. extraterrestrial meteoroids that enter the atmosphere. ANSWER: C 35. Middle latitude storms are also known as a. anticyclones. b. hurricanes. c. extratropical cyclones. d. tornadoes. ANSWER: C 36. At night, when the weather is extremely cold and dry, a. atmospheric pressure increases with increasing altitude. b. atmospheric pressure remains constant with increasing altitude. c. atmospheric pressure decreases with increasing altitude. d. atmospheric pressure first increases, then decreases with increasing altitude. ANSWER: C 37. In the middle latitudes of the Northern Hemisphere, surface winds tend to blow ____ and ____ around an area of surface low pressure. a. clockwise; inward b. clockwise; outward c. counterclockwise; inward d. counterclockwise; outward ANSWER: C 38. The difference in altitude (i.e., the thickness) is greatest in the layer bounded by a. 1 mb and 10 mb. b. 101 mb and 110 mb. c. 1001 mb and 1010 mb. d. impossible to determine ANSWER: A 39. On a weather map, sharp changes in temperature, humidity, and wind direction are marked by a. a front. b. an anticyclone. c. a ridge. d. blowing dust. ANSWER: A 13 40. Which of the following is MOST likely associated with fair weather? a. high pressure area b. low pressure area c. a cold front d. a warm front ANSWER: A 41. Which relates to weather rather than climate? a. The average temperature for the month of January is 28°F. b. The lowest temperature ever recorded in Frozenlake, Minnesota is -57°F. c. The foggiest month of the year is December. d. I like the warm, humid summers. e. Outside it is cloudy and snowing. ANSWER: E 42. In an average year, more people die from ____ than from any other natural disaster. a. lightning b. earthquakes c. tornadoes d. flash floods and flooding e. droughts ANSWER: D 43. At the 500 mb level, the amount of oxygen inhaled in a single breath is ____ of that inhaled at sealevel. a. about the same b. about one-quarter c. about one-half d. about three-quarters ANSWER: C 44. The ozone hole is an actual hole in the atmosphere, a region of complete vacuum. a. true b. false ANSWER: B 45. Ozone in the stratosphere a. is a health hazard for people with respiratory illnesses. b. protects life from harmful ultraviolet radiation. c. is one of the main ingredients of photochemical smog. d. b and c ANSWER: B 14 46. If you were to take a breath of pure oxygen, from a tank, you'd be getting about ____ the amount of oxygen you'd get by taking a normal breath of our atmosphere. a. one-fifth b. half c. twice d. three times e. five times ANSWER: E 47. Standing at the top of a tall mountain, a breath of air would contain a lot fewer molecules than a breath of air taken at sea level. But the proportion of oxygen in the two breaths of air, relative to the other constituents, would remain the same. a. true b. false ANSWER: A 48. There is a lot of mixing and overturning of air in which of the following atmospheric layers? a. stratosphere b. troposphere c. mesosphere ANSWER: B 49. As a radiosonde balloon ascends through the atmosphere, the balloon a. contracts. b. expands. c. maintains a constant pressure. ANSWER: B 50. Atmospheric storm systems can be a. only a few meters wide. b. about a kilometer wide. c. several hundred kilometers wide. d. all of the above ANSWER: D Essay Exam Questions 1. Compare and contrast the Earth’s atmosphere with the atmospheres of two other planets. 2. Describe the various types of storms found in the earth's atmosphere. Can you find any correlation between storm size and storm duration? What factors might determine a storm's severity? 15 3. What instruments are used in meteorology? What role did the discovery of instruments play in the emergence of the science of meteorology? 4. Briefly describe some of the historical events that helped meteorology progress as a natural science from Aristotle to the present day. 5. Under what circumstances might a person breathe stratospheric air? How often is it likely to happen in a student's lifetime? 6. What causes air pressure? Why does air pressure decrease with increasing altitude? 7. Describe some of the processes that release and remove carbon dioxide from the atmosphere. Is there any evidence that suggests that these processes are not in balance? 8. There is currently concern that the amount of ozone in the stratosphere may be decreasing. Why would a decrease in ozone concentration be important? Describe some of the effects that a decrease in ozone concentration might have. 9. If the air temperature at the surface (0 feet) is 60°F, what would be the approximate air temperature at an altitude of 10,000 feet, assuming an average atmospheric lapse rate of 3.6°F per 1000 feet? 10. Draw a diagram showing how air temperature normally changes with height. Begin at the ground and end in the upper thermosphere. Be sure to label the four main layers. Give one important characteristic of each layer. Where on your diagram would the top of Mt. Everest, the ozone layer, and the ionosphere be found? 11. What are the principal gaseous components of the earth's atmosphere? Where do scientists believe these gases came from? 12. Why is there very little water vapor above the tropopause? 13. What information might you find on a surface weather map that is not readily apparent on a satellite photograph? What information could a satellite photograph provide that a surface chart could not? 14. Explain briefly why it is possible to transmit AM radio waves over larger distances at night than during the day. 15. Describe the relationship between gravity and weight.