Physical Science lab on chemical reactions 1
advertisement

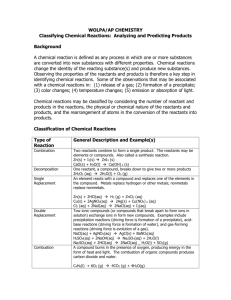
Physical Science Lab on Chemical Reactions There are 5 stations with 5 potential reactions. Make a table so that at each station you can answer the following questions in your lab notebook: a. Did a chemical reaction take place? If so, how did you know ? Change in color, energy released or absorbed, change in smell, gas produced? b. Formula of reactant(s) c. Formula of product(s) d. Type of chemical reaction? Synthesis? Decomposition? Single replacement? Double replacement? Oxidation/reduction? e. Write the balanced equation for the reaction (including all physical states). Station 1: Place 10 drops of silver nitrate into a small test tube. Add 10 drops of sodium chloride to the same test tube. Word equation: silver nitrate plus sodium chloride yields silver chloride plus sodium nitrate Station 2: Place 200mL water in a beaker and place on the hot plate. Wait for steam to be released from the surface Word equation: water becomes steam Station 3: Place a small piece of zinc into a small test tube. Add enough dilute hydrochloric acid (HCl) to just cover the piece of zinc. Word equation: zinc plus hydrochloric acid yields zinc chloride plus hydrogen gas Station 4: Place a small piece of magnesium ribbon (about 10 mm long) into a small test tube. Add enough copper (II) sulfate to just cover the ribbon. Set aside for 15 minutes. Examine the contents of the test tube. If no change is observed, notify your teacher. Word equation: Aqueous Copper (II) sulfate reacts with magnesium to produce aqueous magnesium sulfate and copper Station 5: Place 20 drops of hydrogen peroxide into a small test tube. Add a small amount of manganese (IV) oxide to the tube (just enough to cover the bottom of the test tube). HINT: manganese (IV) oxide is a catalyst. It is not a reactant or product, but it speeds up the rate of the reaction. A catalyst is indicated in a chemical reaction by writing the formula of the catalyst over the arrow. Word equation: Aqueous hydrogen peroxide produces water and oxygen. Questions to answer in your lab notebook for the next class: 1.Name five types of chemical reactions. 2.What is a reactant in a chemical reaction? 3.What is a product in a chemical reaction? 4.Name four ways you can tell a chemical reaction hastaken place. 5.What does the symbol mean in a chemical equation? 6.Give the four symbols for physical states of reactants and products, and tell what each means. 7. Why must chemical equations be balanced? Conclusion: Was the purpose of this lab achieved? What evidence do you have to support your answer?