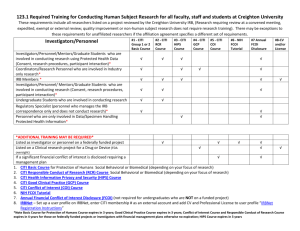
Basic - CITI
advertisement

Conflicts of Interest (COI) Basic Course Terms and Conditions Please select the module(s) that your organization would like to use. Module Name Description Module 1: Financial Conflicts of Interest: Overview, Investigator Responsibilities, and COI Rules Module 2: Institutional Responsibilities as They Affect Investigators Module 3: OrganizationSpecific Policies (basic level) Modules 1 and 2 will provide training on the PHS/NIH regulations related to financial conflicts of interest (PHS regulations on Responsibility of Applicants for Promoting Objectivity in Research for which PHS Funding is Sought [2 C.F.R. Part 50, Subpart F] and Responsible Prospective Contractors [45 C.F.R. Part 94]) that were revised in 2011. These modules will serve as the core training for this course. Modules 1 and 2 are mandatory. Module 3 is an optional organizationspecific module that the CITI Program can create using the information you provide. Does your Yes: organization want to create an organizationspecific module? Does your Yes: organization want to make Module 4 mandatory for the learner? Does your Yes: organization want to make Module 5 mandatory Module 4: Conflicts of Commitment and Conscience Module 4 is an optional module available to all learners that provides training on conflicts of commitment, conscience, and institutional conflicts of interest. If your organization prefers, the CITI Program system can make the module mandatory for the learner. Module 5: Institutional Conflicts of Interest Module 5 is an optional module on institutional conflicts of interest that is primarily designed for institutional administrators. If your organization prefers, the CITI Program system can make the module mandatory for the learner. Next No: No: No: for the learner? As a part of the CITI Program’s COI Basic Course, your organization has the option of creating Module 3, which will provide learners with information about your organization’s COI policies. If you do not wish to add Module 3, please proceed to Part 8 of this form. Submission Criteria Information about your organizational policies must be submitted to the CITI Program in a finalized form. Your organization is responsible for the accuracy of the information sent to the CITI Program. The information should be thoroughly reviewed by your organization prior to submission. The CITI Program will only accept content that is presented in a (.doc, .rtf, or .docx) format. The CITI Program CANNOT accept: a. PDFs, handwritten, scanned, or faxed materials b. Documents that are not final or include notes, comments, strikethroughs or any other type of editorial feedback If you wish to display images, videos, graphs, charts, and case studies, please be sure to include the intended location within the module and the presentation style (for example, hyperlink, image within module, etc.) for each supplemental learning material. It is the responsibility of the organization to ensure that all supplemental learning materials and hyperlinks within the module remain active and updated. How To Submit Your Completed Content All organization-specific content must be sent via e-mail to citisupport@med.miami.edu as a finalized document. You will be sent a unique case number to confirm the receipt of your content. Please retain this case number for your records. Expected Completion Timeframe Upon receiving your form, your request will be placed in the CITI Program support queue for the next available technician to set up. Requests will be handled based on the order in which they are received. Next The CITI Program will work to process your request in an expedient manner. Keep in mind that the timeframe for the completion of your COI Course will be in direct relation to when organization-specific content is submitted. Please note: if your organization-specific module is greater than 5,000 words or has multiple supplemental learning tools, this may require a longer time until the content upload is completed. To check the status of your request, contact the CITI Program Help Desk at (305) 243-7970 or citisupport@med.miami.edu with your case number. Next Approval Policy Once your setup is complete, a CITI Program representative will contact your organization’s delegated administrator (that you assign on the last page of this form) for final approval. In order for the course to be available to your learners, the delegated administrator must provide written consent via e-mail within seven (7) business days. Additional instructions on approval will be provided at that time. During this time, it is the organization’s responsibility to review the module’s content for accuracy. The CITI Program is not responsible for the content provided and uploaded to create the module. The organization providing the content must check for accuracy. Please note: Only your delegated administrator can approve your organization-specific module before it goes live. Updates to the Organization-specific Module As with all other customized modules, your organization is responsible for notifying the CITI Program about any changes to the module’s content, including links. The delegated administrator within your organization should, at a minimum, review the module annually and notify the CITI Program accordingly. Fees All requests to add or modify the organization-specific module (Module 3) will be subject to our current custom module programming fee(s) of $100/per hour. There will be no fee(s) for CITI Program subscribers to add modules 1, 2, 4, or 5 to their existing curriculum. New subscribers will be subject to the CITI Program’s subscription fee in order to have access to the COI Basic Course. Acceptance I agree to all terms and conditions [ ] ___________________________________________________________ Please Print Name Above to Indicate Approval Next Module Set-Up Information Name of Organization Please provide the name of your organization. If your organization is already affiliated with the CITI Program, provide the name as it appears on your organization’s page. If you represent a new organization, the CITI Program’s Billing Office will contact you as a part of the set-up process. Access to the COI Basic Course is part of the organizational base subscription fee. Organization Name: Is your organization currently Yes: No: If “No,” please be aware that a member of the CITI Program’s affiliated with the CITI Program? Billing Office will contact you within five (5) business days of receiving this form. Please Provide Your Contact Information Name: Position in the Organization: Phone: Fax: E-mail: Will this person be responsible for reviewing and updating the information in the Organization-specific Module? Will this person be responsible for payment, if the organization is not already affiliated with the CITI Program? Yes: No: (If “no,” please provide name and contact of person responsible for ongoing updates.) Name: Phone: E-mail: Yes: No: (If “no,” please provide name and contact of person responsible for payment.) Name: Phone: E-mail: Please Indicate The Person Responsible For The COI Program At Your Organization If there is more than one individual, please copy and complete one table per individual. Name: Position in the Organization: Phone: Fax: E-mail: Next Module Content Information Part 1 Introduction Introduce your organizational policies. This section may also contain a statement from your organization’s official. The CITI Program has provided some recommended sections that should be included in the custom module; however, your organization may provide additional content as appropriate. Sample items include: A summary of the organization’s commitment regarding COIs The organizational training policy regarding financial conflicts of interest (FCOIs) A general overview of the organization’s training requirements for investigators, staff, and students A description of the policies for awardees versus sub-awardees, and note if there are any requirements for non-PHS funded investigators and staff An overview of re-training requirements (e.g., four-year minimum or more often if required by the organization) Organizational plan to comply with the 2011 Final Rule (revised PHS regulations) Provide your introductory paragraph text below: Learning Objectives It is important to provide measurable learning objectives that represent the focus of this organizationspecific module. The learning objectives should incorporate strong action verbs. For example: By the end of this module, you should be able to: Discuss the regulatory definitions associated with financial conflicts of interest (FCOIs) as defined by the 2011 Final Rule (revised PHS regulations) Describe the organization’s policies related to COIs Identify the individuals responsible for the COI Program at your organization Provide your learning objectives below: Please use a separate row for each objective. Space for three (3) learning objectives has been provided; however, additional objectives may be included. Learning Objective 1: Learning Objective 2: Learning Objective 3: Next Part 2 Organizational Policies and Procedures This section is intended to provide general statements regarding your organizational policies and procedures that serve as an overview. In addition, it is recommended that this section include commentary regarding the organization’s response to the requirement that the FCOI policy be available via a publicly accessible website (unless the organization has no current web presence). This section should also contain an overview of your organization’s definitions as they relate to the FCOI policy and procedures. An in-depth review of portions of the organizational policies and procedures will follow in subsequent sections. The CITI Program encourages you to have learners visit your website directly via the links (provided in this section) to your policies and procedures. This will also allow learners to bookmark the website(s). Referring the learners to your organization’s website(s) as the primary source for the review of policy/procedure information also means that any changes to those policies/procedures do not have to be communicated back to the CITI Program unless they are no longer consistent with the overview statements listed here. URLs should be provided as a list at the end of the section, but you may reference the URLs by name of page within the body of the narrative. When you provide the URL, please put the name of the page, as you would like it to appear in the section. Provide the general statements regarding your organizational policies and procedures below: Definitions The CITI Program will insert the following standard definitions presented by the NIH; however, if your organization defines any of the following differently, please insert your revised definition in the space that follows. The CITI Program recommends that you include the following information within this section: What is your organization’s definition of FCOI? What is your organization’s definition of significant financial interest (SFI)? What is your organization’s definition of “employee’s institutional responsibilities?” Investigator means the project director or principal investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research funded by the National Institutes of Health (NIH), or proposed for such funding, which may include, for example, collaborators or consultants. Institutional responsibilities means an Investigator's professional responsibilities on behalf of the organization, and as defined by the organization in its policy on financial conflicts of interest, which may include for example: activities such as research, research consultation, teaching, professional practice, institutional committee memberships, and service on panels such as Institutional Review Boards or Data and Safety Monitoring Boards. Significant Financial Interest (SFI) is (1) A financial interest consisting of one or more of the following interests of the investigator (and those of the Investigator's spouse and dependent children) that reasonably appears to be related to the Investigator's institutional responsibilities: Next (i) With regard to any publicly traded entity, a significant financial interest exists if the value of any remuneration received from the entity in the twelve months preceding the disclosure and the value of any equity interest in the entity as of the date of disclosure, when aggregated, exceeds $5,000. For purposes of this definition, remuneration includes salary and any payment for services not otherwise identified as salary (e.g., consulting fees, honoraria, paid authorship); equity interest includes any stock, stock option, or other ownership interest, as determined through reference to public prices or other reasonable measures of fair market value; (ii) With regard to any non-publicly traded entity, a significant financial interest exists if the value of any remuneration received from the entity in the twelve months preceding the disclosure, when aggregated, exceeds $5,000, or when the investigator (or the investigator's spouse or dependent children) holds any equity interest (e.g., stock, stock option, or other ownership interest); or (iii) Intellectual property rights and interests (e.g., patents, copyrights), upon receipt of income related to such rights and interests. Investigators also must disclose the occurrence of any reimbursed or sponsored travel (i.e., that which is paid on behalf of the Investigator and not reimbursed to the investigator so that the exact monetary value may not be readily available), related to their institutional responsibilities, provided, however, that this disclosure requirement does not apply to travel that is reimbursed or sponsored by excluded sources provided in regulation. A financial conflict of interest (FCOI) is an SFI that could directly and significantly affect the design, conduct, or reporting of NIH-funded research. Senior/key personnel means the project director/principal investigator (PD/PI) and any other person identified as senior/key personnel by the organization in the grant application, progress report, or any other report submitted to the PHS by the organization under the regulation. If relevant, please provide your organization’s modified definitions below: Title of Web Page Title of Web Page Title of Web Page Title of Web Page URL URL URL URL Next Part 3 Investigator Disclosure Requirements This section is intended to provide the learner with a detailed review of the organizational policies and procedures associated with investigator disclosure requirements. The CITI Program has provided some guiding instructions and questions to consider as your organization develops the narrative portion for this section. Describe the organizational policy on SFI reporting requirements (include, for example, the thresholds for reporting SFI). What is the mechanism of disclosure? What are the elements of the disclosure? When and how often are investigators required to disclose? To whom or to what office does the investigator disclose the SFI? Is there a web form for employees to complete? (Location of the web form, provide the URL.) What phone number can employees call for assistance with completing the disclosure form? How long should records be maintained? Provide the narrative related to your organization’s disclosure requirements below: Next Part 4 Organization Assessment Responsibilities This section is intended to provide the learner with a detailed description of the process the organization uses to assess SFIs. The CITI Program suggests, at a minimum, the inclusion of information related to the following: How does your organization determine whether SFIs are related to research? (Describe the process.) How does your organization determine if the research-related SFIs create an FCOI? How does you organization address sub-recipient investigators? Provide the narrative describing your organization’s assessment responsibilities and procedures below: Organizational Management Plan for FCOIs This section is intended to provide the learner with an overview of management plans as they relate to the 2011 Final Rule (revised PHS regulations) as well as important information associated with the organization’s management plan. The following questions are suggested for inclusion in your organization’s narrative. What is a management plan? Who at your organization will develop the management plan? What are the required elements of your organization’s management plan? Who reviews and approves the FCOI management plans? Provide the narrative describing your organization’s assessment responsibilities and procedures below: Next Part 5 Organizational Reporting Requirements Organizations must report FCOIs of their employees to the NIH via the eRA Commons FCOI Module. This section is intended for your organization to describe this process. Note that annual reporting is required as well as reporting during the period of award within sixty (60) days of identifying a new FCOI. Describe the elements that at minimum will be reported to the PHS. It is recommended that bullets be used to list the items, which will be reported. FCOI Disclosure (Public Accessibility) FCOIs of senior/key personnel discovered during the assessment process must be either displayed on the organization’s website or made available by a written response to any requestor within five (5) business days of a request. Consider including the following: How the information will be made available? (Will it be posted on a website? If so, provide the URL. If it will be provided via written response, detail that process.) The elements that will be displayed on the website. (2011 Final Rule/revised PHS regulations require that at a minimum the investigator’s name, title, and role with respect to the research project be listed along with the name of the entity in which the SFI is held, the nature of the SFI, and the approximate dollar value of the SFI or a statement that the interest is one whose value cannot be readily determined through reference to public prices or other reasonable measures of fair market value.) Address the organization’s disclosure policy and procedures below: Part 5A SFI/FCOIs Identified Post Disclosure (Retrospective Review) If the organization discovers a SFI/FCOI that was not identified in the disclosure and assessment process, the organization is required to conduct a retrospective review to determine if the SFI is an FCOI and if the FCOI has led to the collection and/or the reporting of biased data. The purpose of this section is to provide the learner with information related to the organization’s policy and procedures related to SFI/FCOIs identified post disclosure. The CITI Program recommends inclusion of information related to the following questions. How does your organization define “retrospective review”? The regulatory requirement is that the review be within 120 days. Is the organizational policy the same? Who will conduct the retrospective review at your organization? What are the required elements of the retrospective review? What other elements might the retrospective review include? Please provide the narrative related to SFI/FCOI identified post disclosure procedure, including retrospective review, for the organization below: Next Part 5B Identification of Bias If the retrospective review concludes that the research may have been biased as a consequence of the unreported FCOI, the organization is required to submit a mitigation report to the PHS. This section is intended for you to describe the mitigation reporting. The CITI Program recommends inclusion of information related to the following questions: How does your organization define “institutional mitigation report”? What are the elements of the mitigation report at the organization? (Note that the 2011 Final Rule/revised PHS regulations require that the report address the impact of the bias on the research project and the actions the organization has taken, or will take, to eliminate or mitigate the effect of the bias.) Who will review and approve the mitigation report? Describe your organization’s mitigation reporting process here: Next Part 6A Additional Information If needed, provide any additional information related to your organization’s conflict of interest policies for your learners. Please provide the additional information below: Part 6B Summary This section is intended to provide concluding comments for your organization’s learners along with any additional information. The CITI Program recommends the following: A summary statement that provides an overview of the content presented in the module Additional organizational links Links to external sources (PHS and other entities) A statement directing learners to the quiz/assessment (if your organization elects to quiz/assess learners, see next section). Provide the summary narrative below: Next Part 7 Quiz/Assessment Does your organization want to quiz your investigators on the materials included in this module? Yes: No: If Yes: If No: Nothing further is required in this It is recommended that five (5) multiplesection. Note that the CITI choice questions be provided. Program will include an attestation o 4-5 potential responses for each notice (“I have reviewed this module question, but only one true correct and the applicable institutional answer. documents”) that learners must o Feedback to the learner describing check at the end of the module to why the correct answer is correct. complete the process. o Avoid negative constructions of questions (for example using “except” or double negatives). o Questions should be answerable based on either the content within the module or that appears as a direct result of links within the module. It is not advisable to quiz or assess on links on secondary pages. Provide questions, answer choices, and feedback here (you may copy the rows for as many questions as your organization would like to provide; remember to mark the correct answer): Question Stem: Answer Choice 1: Answer Choice 2: Answer Choice 3: Answer Choice 4: Feedback for learner: Question Stem: Answer Choice 1: Answer Choice 2: Answer Choice 3: Answer Choice 4: Feedback for learner: Question Stem: Answer Choice 1: Answer Choice 2: Answer Choice 3: Answer Choice 4: Feedback for learner: Next Part 8 Learner Group Set-Up Information Delegated Administrator Please provide the contact information of your delegated administrator. This is the person who will be contacted to approve the final course. Name of Delegated Administrator E-mail Address CITI Program Member ID # Located on top left corner of screen when logged into citiprogram.org The CITI Program default settings for the COI Course are listed below. If you would like to customize the settings, please provide your information in the chart labeled Custom Settings. Course Name That Appears To Your Learners: Passing Score: Default Settings Conflicts of Interest (COI) Basic Course 80% Expiration: 4 years Enrollment Question Answer 1: Answer 2: Would you like to take the Conflicts of Interest (COI) Basic Course? Yes (learner is enrolled in the course) No (no action taken from response) Custom Settings – If you do not edit this section, the default settings above will apply. Course Name That Appears To Your Learners: Passing Score: Expiration: Enrollment Questions Answer 1: Answer 2: Next End of Form