Vinyl Acetate SOP
advertisement

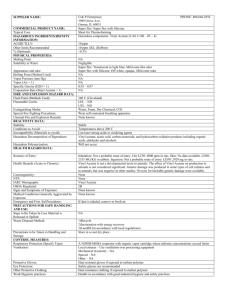
Standard Operating Procedure Vinyl acetate Settlement Class: Peroxide Forming Chemicals Print a copy and insert into your Laboratory Safety Manual and Chemical Hygiene Plan. Refer to instructions for assistance. Department: Chemistry Date SOP was written: 11/6/2012 Date SOP was approved by PI/lab supervisor: Principal Investigator: Richmond Sarpong Internal Lab Safety Coordinator/Lab Manager: Lab Phone: 1/13/2013 Rebecca Murphy 510-643-2485 Office Phone: 510-643-6312 Emergency Contact: Richmond Sarpong 626-644-2407 Location(s) covered by this SOP: Latimer 834, 836, 837, 838, 839, 842, 844, 847, 849, 907 (Name and Phone Number) (Building/Room Number) Type of SOP: Vinyl acetate ☒ Process ☒Hazardous Chemical 1 SOP Template developed by The UC Center for Laboratory Safety ☐ Hazardous Class Date: 9/1/2012 Purpose Vinyl acetate or vinyl acetate monomer (VAM) is an acute and chronic toxin. Possible carcinogen. Extremely flammable liquid. Irritating to eyes and respiratory system. Slightly irritating to the skin. Keep away from heat, sparks and flame. Avoid exposure, do not breathe vapor or mist. Avoid contact with eyes, skin and clothing. May cause target organ damage. Vinyl acetate can be polymerized to make polyvinyl acetate Physical & Chemical Properties/Definition of Chemical Group CAS#: 108-05-4 Class: Highly flammable liquid, toxic, possible carcinogen Molecular Formula: CH3COOCH=CH2 Form (physical state): Liquid Color: Colorless to light yellow Boiling point: 72.2°C Potential Hazards/Toxicity Vinyl acetate is a highly flammable liquid. Toxic. May be carcinogenic. Vinyl acetate has a threshold limit value - time weighted average (TWA) of 30 mg/m³ 8 hours and 10 ppm 8 hours. May cause gastrointestinal irriation with nausea and vomiting. If inhaled, may cause respiratory tract irritation with burning pain, shortness of breath. Exposure to skin may cause irritation. May cause respiratory irritation. May be harmful if inhaled, absorbed through the skin, in contact with eyes, or if swallowed Inflammation of the eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Vinyl acetate 2 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 Vinyl acetate has the following toxicity data: Acute oral toxicity (LD50): 1613 mg/kg [Mouse]. Acute dermal toxicity (LD50): 2335 mg/kg [Rabbit]. Acute toxicity of the gas (LC50): 2828.4 ppm 4 hour(s) [Rat]. Acute toxicity of the vapor (LC50): 1550 ppm 4 hour(s) [Mouse]. Personal Protective Equipment (PPE) Respirator Protection Respirators should be used only under any of the following circumstances: As a last line of defense (i.e., after engineering and administrative controls have been exhausted). When Permissible Exposure Limit (PEL) has exceeded or when there is a possibility that PEL will be exceeded. Regulations require the use of a respirator. An employer requires the use of a respirator. There is potential for harmful exposure due to an atmospheric contaminant (in the absence of PEL) As PPE in the event of a chemical spill clean-up process Lab personnel intending to use/wear a respirator mask must be trained and fit-tested by EH&S. This is a regulatory requirement. Hand Protection Gloves must be worn with when handling peroxide-forming chemicals. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Wash and dry hands. Nitrile gloves are recommended. NOTE: Consult with your preferred glove manufacturer to ensure that the gloves you plan on using are compatible with vinyl acetate. For glove selection, go to: http://ehs.berkeley.edu/hs/63-laboratory-safety/94-glove-selection-and-usage.html Eye Protection Vinyl acetate 3 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 Safety glasses with side shields or tightly fitting safety goggles. Use goggles and face shield (8-inch minimum) when appropriate. Only use equipment for eye protection tested and approved under appropriate government standards such as ANSI Z 87.1, NIOSH (US), or EN 166(EU). Skin and Body Protection Flame resistant lab coats must be worn and be appropriately sized for the individual and buttoned to their full length. Laboratory coat sleeves must be of sufficient length to prevent skin exposure while wearing gloves. As outlined in Policy 905 personnel should also wear full length pants, or equivalent, and close-toed shoes. Full length pants and close-toed shoes must be worn at all times by all individuals that are occupying the laboratory area. The area of skin between the shoe and ankle should not be exposed. Hygiene Measures Wash thoroughly and immediately after handling. Wash hands before breaks and at the end of workday. Remove contaminated clothing and wash before reuse. Engineering Controls Work with peroxide-forming chemicals must be conducted in a fume hood unless other controls are designated in the Protocol/Procedure section. Sash height must be kept low to avoid escaping fumes and provide explosion barrier. Use supplemental explosion protective equipment like a blast shield where appropriate to protect from explosions in the case of peroxide detonation. Make sure all equipment and lines are grounded. First Aid Procedures Notify supervisor and EH&S immediately. Follow up with a call to 510-642-9090 to report the incident. If inhaled Move into the fresh air immediately and give oxygen. If not breathing give artificial respiration. Get medical attention immediately. In case of skin contact Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention immediately. In case of eye contact Check for and remove any contact lenses. Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. Seek immediate medical attention and continue eye rinse during transport to hospital. Vinyl acetate 4 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 If swallowed Do NOT induce vomiting unless directed by SDS. Never give anything by mouth to an unconscious person. Seek medical attention immediately. Special Handling and Storage Requirements Working alone Certain extremely hazardous operations should not be performed if the PI or Lab Safety Contact(s) are not present. Never work alone with extremely hazardous materials/operations. See the Protocol/Procedure section for specific prohibitions (if any) on working alone. Precautions for safe handling: Avoid contact with skin and eyes and inhalation. Avoid dust formation or breathing vapors, mist, or gas. Use only with adequate ventilation or respiratory protection. Keep away from heat or sources of ignition. Prevent any build-up of electrostatic charge. Conditions for safe storage: Conditions for safe storage: Keep container tightly closed in a cool, dry, and well-ventilated place. Isolate from incompatible materials and conditions. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Light sensitive. Store in original General Precautions Minimize the quantity of peroxides or peroxide forming chemicals in the lab. Label each container with the Date Received, Date Opened and Date Last Tested. Segregate these compounds from incompatible materials. Store away from ignition sources. Protect from flames, static electricity, and sources of heat. Test chemicals for peroxide before any distillation or purification of peroxide forming chemicals. NEVER distill potential peroxide-forming chemicals to dryness. Always leave a minimum of 20% still bottoms. When possible, add a non-volatile organic compound (such as mineral oil) to dilute any peroxides remaining after distillation. Use extreme caution before concentrating or purifying peroxide forming chemicals as most explosions occur during these processes. Wear proper personal protective equipment, including safety eyewear and face shields, when working with peroxide forming chemicals. Minimize peroxide formation in ethers by storing in tightly sealed containers in a cool place in the absence of light. Vinyl acetate 5 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 If solids or crystals are observed in either the liquid or around the cap of peroxide forming chemicals, do not open or move the container but contact EH&S for disposal. Visual Inspection: Visually inspect all peroxide-forming chemicals before any operation. Containers that exhibit any unusual visual characteristics, such as the examples listed below, should be assumed to contain dangerous levels of peroxides and should not be disturbed. Notify EH&S, who will assist in the further evaluation. If there is any doubt about the safety of handling a chemical container, notify EH&S immediately. For liquid chemicals, look for crystallization (around the cap or in the liquid), visible discoloration, or liquid stratification. Diethyl ether is commonly sold in steel containers which prevents visual inspection of the liquid. Therefore, diethyl ether containers whose age and use history are unknown should be assumed to contain dangerous levels of peroxides and should not be disturbed. For solid chemicals (potassium metal, potassium amide, and sodium amide), look for discoloration and/or formation of a surface crust (for example, potassium metal forms a yellow or orange superoxide at the surface). Evaluation of alkali metals and their amides is based on visual criteria only. These substances react strongly with water and oxygen, and the standard operating procedures for pyrophoric compounds should be followed for these chemicals. Materials not passing visual inspection are considered to be high risk and will have to be disposed of by special means (limit handling and movement; notify EH&S). Only chemicals that pass visual inspection should be tested. Testing for Peroxides Note: Never try to force open a rusted or stuck cap on a container of a peroxide-forming chemical. There is a great deal of uncertainty regarding the concentration at which peroxides pose a hazard to researchers. Various sources suggest that the minimum hazardous concentration of peroxides in organic solution is in the range 0.005 - 1.0% (5010000 PPM). In most safety literature, a conservative concentration of 100 PPM peroxides is used as a control point. By the end of the expiration date (as indicated in Table 2) for a particular peroxide forming chemical, the person using the chemical should either dispose of it or test it for peroxide content. Any container found to have a peroxide concentration greater than or equal to 100 PPM should be disposed of (call EHS&S for assistance). Materials which are older than the suggested shelf life but have been tested and have no detectable peroxides or peroxide concentrations less than 100 PPM may be retained but should be tested at frequent intervals (see Table 2). All chemicals which are to be distilled must be tested Vinyl acetate 6 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 prior to distillation regardless of age. Important note: Researchers should never test containers of unknown age or origin. Older containers are far more likely to have concentrated peroxides or peroxide crystallization in the cap threads and therefore can present a serious hazard when opened for testing. Please read section below on managing older containers. INSERT #17 There are several methods that are commonly used to detect for peroxides in the laboratory. Perhaps the most convenient method is the use of peroxide test strips which are manufactured by Aldrich and several other suppliers. These strips are simple to use and can be obtained from EHS&S. For volatile organic chemicals, the test strip is immersed in the chemical for 1 second; then the tester breathes slowly on the strip for 15-30 seconds or until the color stabilizes. The color is then compared with a colorimetric scale provided on the bottle. Strips that offer a 1-100 PPM peroxide range are useful for determining if the material is below the control point of 100 PPM. Other testing methods are available. Contact EHS&S for more information. Purchasing Ideally, purchases of peroxidizable chemicals should be restricted to ensure that these chemicals are used up completely before they can become peroxidized. This requires careful experiment planning on behalf of researchers. Researchers should purchase no more material than is needed to complete an experiment within the chemical’s safe shelf life. INSERT #17 Storage and Shelf Life Peroxides tend to form in materials as a function of age. Therefore, it is imperative that researchers are keenly aware of the age of their peroxidizable chemicals. Researchers must date each container of peroxidizable chemical upon arrival in the laboratory. Containers must be dated again when opened for the first time. Additional dates of testing should be added in certain cases (see below). Special labels as depicted below make dating of the containers convenient. These labels are available free of charge from EHS&S. For uninhibited vinyl actetate (on List C), materials: 24 hours and Inhibited: 12 months2 the safe shelf life. Suggested time limits are given for retention or testing of these compounds. However, it must be noted that these shelf life durations are minimum criteria; many other references recommend more frequent testing for peroxides. Peroxide forming chemicals should be stored in their original manufacturer’s container whenever possible. This is very important in the case of diethyl ether because the iron in the steel containers that this material is shipped in acts as a Vinyl acetate 7 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 peroxide inhibitor. In general, peroxidizable chemicals should be stored in sealed, air-impermeable containers and should be kept away from light (light can initiate peroxide formation). Dark amber glass with a tight fitting cap is appropriate Label for peroxide forming chemicals Warning: May Form Explosive Peroxides Store in tightly closed original container. Avoid exposure to light, air and heat. If crystals, discoloration or layering is visible, do not move or open and contact EHS&S immediately. Check for peroxides before distilling or concentrating. This Chemical has a limited shelf life! Date Received Date Opened Test or dispose months after receipt or months after opening. Do not use chemical if >100 PPM peroxides are detected. Test date Test date Peroxides Peroxides PPM PPM Spill and Accident Procedure Chemical Spill Dial 911 Spill – Assess the extent of danger. Help contaminated or injured persons. Evacuate the spill area. Avoid breathing vapors. If possible, confine the spill to a small area using a spill kit or absorbent material. Keep others from entering contaminated area (e.g., use caution tape, barriers, etc.). Dial 911 and 510-642-9090 for assistance. Vinyl acetate 8 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 Chemical Spill on Body or Clothes – Remove clothing and rinse body thoroughly in emergency shower for at least 15 minutes. Seek medical attention. Notify supervisor and EH&S immediately. Follow up with a call to 510-642-9090 to report the incident. Chemical Splash Into Eyes – Immediately rinse eyeball and inner surface of eyelid with water from the emergency eyewash station for 15 minutes by forcibly holding the eye open. Seek medical attention. Notify supervisor and EH&S immediately. Follow up with a call to 510-6429090 to report the incident. Medical Emergency Dial 911 Life Threatening Emergency, After Hours, Weekends And Holidays – Dial 911 or go to the nearest emergency room. Note: All serious injuries must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Non-Life Threatening Emergency – Go to the Occupational Health Facility. At all other times report to the nearest emergency room. Note: All serious injuries must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Needle stick/puncture exposure exposure (as applicable to chemical handling procedure) – Wash the affected area with antiseptic soap and warm water for 15 minutes. For mucous membrane exposure, flush the affected area for 15 minutes using an eyewash station. Go to the Occupational Health Facility (Tang Health Center). After hours go to the nearest emergency room. Note: All needle stick/puncture exposures must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Hazardous Waste Disposal Using proper personal protective equipment as outlined above, decontaminate equipment and bench tops using soap and water and properly dispose of all chemical and contaminated disposables as hazardous waste following the guidelines below. General hazardous waste disposal guidelines: Label Waste Vinyl acetate 9 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 Label all containers with the label provided at http://ehs.berkeley.edu/hm/279-new-hazardous-waste-program-hwp.html. See the EH&S Fact Sheet, “Hazardous Waste Management” for general instructions on procedures for disposing of hazardous waste. Dispose of Waste Dispose of regularly generated chemical waste within 6 months. Call EH&S for questions. Management and disposal of old containers Older containers of peroxidizable chemicals, or containers of unknown age or history, must be handled very carefully and should never be opened by researchers. Any peroxidizable chemical with visible discoloration, crystallization or liquid stratification should be treated as potentially explosive. Older steel containers that have visible rust may also be extremely dangerous. If any of these conditions are observed on a peroxidizable chemical or if the origin and age of the container are unknown, do not attempt to move or open the container. Please call EHS&S for assistance. We will arrange to have the container(s) inspected and if necessary will arrange for disposal. Safety Data Sheet (SDS) Location SDS can be accessed online at http://ucmsds.com Vinyl acetate 10 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 Protocol/Procedure Protocol/Procedure for– Vinyl Acetate Preparation Know the location of the nearest fire extinguisher, eyewash, and safety shower before beginning work. Eliminate any incompatible materials from the potential spill area. Never work alone. Make sure there is another worker present who is also trained in the Vinyl acetate SOP. Lab-specific Information Procedure/Use Scale Engineering Controls/Equipment PPE (eye, face, gloves, clothing) Procedure Steps and Special Precautions for this Procedure Vinyl acetate can be used as a reagent in a wide range of organic reactions. For small quantities (<10 mL) a syringe should be used to transfer vinyl acetate from its container to the reaction vessel Vinyl acetate must be used in the fume hood as exposure can resulted in eye irritation and upper respiratory tract irritation. Vinyl acetate is classified as flammable (flash point 7.7 °C) so it should not be used in the presence anything that can create a spark (ex. Bunsen burner, heat gun, etc.) Vinyl acetate should be stored in chemical reagent bottles provided by chemical suppliers. It can be disposed of if it no Eye protection: Wear tight-fitting safety goggles or safety glasses with side shields. Face protection: Wear a face shield if not protected by a fume hood sash. Clothing: Wear flame resistant lab coat; full length pants or equivalent; and close-toed, closeheeled shoes. Gloves: Wear gloves – butyl gloves recommended. Change glove as soon as contaminated. Wear flame resistant lab coat; full length pants <10 ml, 10ml-60 ml or > 60 ml can be added slowly to the reaction vessel For intermediate quantities Vinyl acetate 11 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 (10-60 mL) either a syringe or graduated cylinder may be used to transfer the reagent. longer appears as it should based on the supplier description. It can be added to standard liquid waste. Large quantities (>60 mL) may be transferred using a graduated cylinder. For intermediate quantities (10-60 mL) either a syringe or graduated cylinder may be used to transfer the reagent. Notes Initials of individuals using this procedure Any deviation from this SOP requires approval from PI. NOTE Vinyl acetate 12 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 Any deviation from this SOP requires approval from PI. Documentation of Training (signature of all users is required) Prior to conducting any work with vinyl acetate, designated personnel must provide training to his/her laboratory personnel specific to the hazards involved in working with this substance, work area decontamination, and emergency procedures. The Principal Investigator must provide his/her laboratory personnel with a copy of this SOP and a copy of the SDS provided by the manufacturer. The Principal Investigator must ensure that his/her laboratory personnel have attended appropriate laboratory safety training or refresher training within the last one year. I have read and understand the content of this SOP: Name Signature Identification Date Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter a date. Click here to enter text. Click here to enter text. Vinyl acetate Click here to enter a date. 13 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012 Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Click here to enter text. Click here to enter a date. Vinyl acetate 14 SOP Template developed by The UC Center for Laboratory Safety Date: 9/1/2012